Are we getting enough sulfur in our diet?
- PMID: 17986345
- PMCID: PMC2198910
- DOI: 10.1186/1743-7075-4-24
Are we getting enough sulfur in our diet?
Abstract
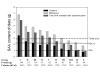
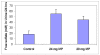
Sulfur, after calcium and phosphorus, is the most abundant mineral element found in our body. It is available to us in our diets, derived almost exclusively from proteins, and yet only 2 of the 20 amino acids normally present in proteins contains sulfur. One of these amino acids, methionine, cannot be synthesized by our bodies and therefore has to be supplied by the diet. Cysteine, another sulfur containing amino acid, and a large number of key metabolic intermediates essential for life, are synthesized by us, but the process requires a steady supply of sulfur.Proteins contain between 3 and 6% of sulfur amino acids. A very small percentage of sulfur comes in the form of inorganic sulfates and other forms of organic sulfur present in foods such as garlic, onion, broccoli, etc.The minimal requirements (RDA) for all the essential amino acids have always been estimated in terms of their ability to maintain a nitrogen balance. This method asses amino acid requirements for protein synthesis, only one of the pathways that methionine follows after ingestion. To adequately evaluate the RDA for methionine, one should perform, together with a nitrogen balance a sulfur balance, something never done, neither in humans nor animals.With this in mind we decided to evaluate the dietary intake of sulfur (as sulfur amino acids) in a random population and perform sulfur balance studies in a limited number of human volunteers. Initially this was done to try and gain some information on the possible mode of action of a variety of sulfur containing compounds (chondroitin sulfate, glucosamine sulfate, and others, ) used as dietary supplements to treat diseases of the joints. Out of this study came information that suggested that a significant proportion of the population that included disproportionally the aged, may not be receiving sufficient sulfur and that these dietary supplements, were very likely exhibiting their pharmacological actions by supplying inorganic sulfur.
Figures





References
-
- Griffith OW. Mammalian sulfur amino acid metabolism: an overview. Methods Enzymol. 1987. - PubMed
-
- Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr. 1994;60:501–509. - PubMed
-
- Wretlind KA, Rose WC. Methionine requirement for growth and utilization of its optical isomers. J Biol Chem. 1950;187:697–703. - PubMed
-
- Rose WC, Wixom RL. The amino acid requirements of man. XIII. The sparing effect of cystine on the methionine requirement. J Biol Chem. 1955;216:753–773. - PubMed
-
- Crim MCM., HN . In: Proteins and Amino Acids, in Modern Nutrition in Health and Disease. Shils ME OJASM, editor. Baltimore, Williams & Wilkins; 1994.
LinkOut - more resources
Full Text Sources

