A photosensory two-component system regulates bacterial cell attachment
- PMID: 17986614
- PMCID: PMC2084327
- DOI: 10.1073/pnas.0705887104
A photosensory two-component system regulates bacterial cell attachment
Abstract
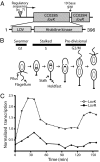
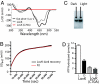
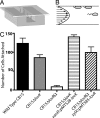
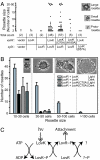
Flavin-binding LOV domains are blue-light photosensory modules that are conserved in a number of developmental and circadian regulatory proteins in plants, algae, and fungi. LOV domains are also present in bacterial genomes, and are commonly located at the amino termini of sensor histidine kinases. Genes predicted to encode LOV-histidine kinases are conserved across a broad range of bacterial taxa, from aquatic oligotrophs to plant and mammalian pathogens. However, the function of these putative prokaryotic photoreceptors remains largely undefined. The differentiating bacterium, Caulobacter crescentus, contains an operon encoding a two-component signaling system consisting of a LOV-histidine kinase, LovK, and a single-domain response regulator, LovR. LovK binds a flavin cofactor, undergoes a reversible photocycle, and displays increased ATPase and autophosphorylation activity in response to visible light. Deletion of the response regulator gene, lovR, results in severe attenuation of cell attachment to a glass surface under laminar flow, whereas coordinate, low-level overexpression of lovK and lovR results in a light-independent increase in cell-cell attachment, a response that requires both the conserved histidine phosphorylation site in LovK and aspartate phosphorylation site in LovR. Growing C. crescentus in the presence of blue light dramatically enhances cell-cell attachment in the lovK-lovR overexpression background. A conserved cysteine residue in the LOV domain of LovK, which forms a covalent adduct with the flavin cofactor upon absorption of visible light, is necessary for the light-dependent regulation of LovK enzyme activity and is required for the light-dependent enhancement of intercellular attachment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

