Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration
- PMID: 17987127
- PMCID: PMC2063465
- DOI: 10.1371/journal.pone.0001150
Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration
Abstract
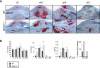
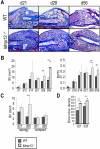
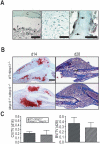
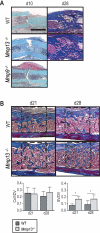
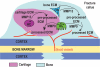
Extracellular matrix (ECM) remodeling is important during bone development and repair. Because matrix metalloproteinase 13 (MMP13, collagenase-3) plays a role in long bone development, we have examined its role during adult skeletal repair. In this study we find that MMP13 is expressed by hypertrophic chondrocytes and osteoblasts in the fracture callus. We demonstrate that MMP13 is required for proper resorption of hypertrophic cartilage and for normal bone remodeling during non-stabilized fracture healing, which occurs via endochondral ossification. However, no difference in callus strength was detected in the absence of MMP13. Transplant of wild-type bone marrow, which reconstitutes cells only of the hematopoietic lineage, did not rescue the endochondral repair defect, indicating that impaired healing in Mmp13-/- mice is intrinsic to cartilage and bone. Mmp13-/- mice also exhibited altered bone remodeling during healing of stabilized fractures and cortical defects via intramembranous ossification. This indicates that the bone phenotype occurs independently from the cartilage phenotype. Taken together, our findings demonstrate that MMP13 is involved in normal remodeling of bone and cartilage during adult skeletal repair, and that MMP13 may act directly in the initial stages of ECM degradation in these tissues prior to invasion of blood vessels and osteoclasts.
Conflict of interest statement
Figures


References
-
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, et al. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. - PubMed
-
- Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. - PubMed
-
- Uusitalo M, Mikkila H, Karma A, Kivela T. Search for autoantibodies against the HNK-1 carbohydrate epitope in the human eye in intermediate uveitis. Acta Ophthalmol Scand. 2000;78:536–538. - PubMed
-
- Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

