A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1
- PMID: 17987129
- PMCID: PMC2048740
- DOI: 10.1371/journal.pone.0001153
A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1
Abstract
Background: The pathogenesis of sepsis is mediated in part by bacterial endotoxin, which stimulates macrophages/monocytes to sequentially release early (e.g., TNF, IL-1, and IFN-gamma) and late (e.g., HMGB1) pro-inflammatory cytokines. Our recent discovery of HMGB1 as a late mediator of lethal sepsis has prompted investigation for development of new experimental therapeutics. We previously reported that green tea brewed from the leaves of the plant Camellia sinensis is effective in inhibiting endotoxin-induced HMGB1 release.
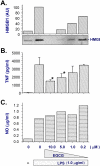
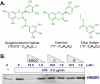
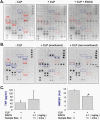
Methods and findings: Here we demonstrate that its major component, (-)-epigallocatechin-3-gallate (EGCG), but not catechin or ethyl gallate, dose-dependently abrogated HMGB1 release in macrophage/monocyte cultures, even when given 2-6 hours post LPS stimulation. Intraperitoneal administration of EGCG protected mice against lethal endotoxemia, and rescued mice from lethal sepsis even when the first dose was given 24 hours after cecal ligation and puncture. The therapeutic effects were partly attributable to: 1) attenuation of systemic accumulation of proinflammatory mediator (e.g., HMGB1) and surrogate marker (e.g., IL-6 and KC) of lethal sepsis; and 2) suppression of HMGB1-mediated inflammatory responses by preventing clustering of exogenous HMGB1 on macrophage cell surface.
Conclusions: Taken together, these data suggest a novel mechanism by which the major green tea component, EGCG, protects against lethal endotoxemia and sepsis.
Conflict of interest statement
Figures





References
-
- Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. - PubMed
-
- Yin K, Gribbin E, Wang H. Interferon-gamma inhibition attenuates lethality after cecal ligation and puncture in rats: implication of high mobility group box-1. Shock. 2005;24:396–401. - PubMed
-
- Fink MP, Payen D. The role of nitric oxide in sepsis and ARDS: synopsis of a roundtable conference held in Brussels on 18–20 March 1995. Intensive Care Med. 1996;22:158–165. - PubMed
-
- Vincent JL, Zhang H, Szabo C, Preiser JC. Effects of nitric oxide in septic shock. Am J Respir Crit Care Med. 2000;161:1781–1785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

