Steric and electrostatic effects in DNA synthesis by the SOS-induced DNA polymerases II and IV of Escherichia coli
- PMID: 17988102
- PMCID: PMC2555966
- DOI: 10.1021/bi700851z
Steric and electrostatic effects in DNA synthesis by the SOS-induced DNA polymerases II and IV of Escherichia coli
Abstract
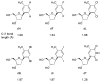
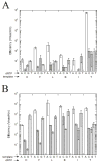
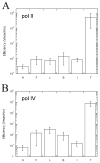
The SOS-induced DNA polymerases II and IV (pol II and pol IV, respectively) of Escherichia coli play important roles in processing lesions that occur in genomic DNA. Here we study how electrostatic and steric effects play different roles in influencing the efficiency and fidelity of DNA synthesis by these two enzymes. These effects were probed by the use of nonpolar shape analogues of thymidine, in which substituted toluenes replace the polar thymine base. We compared thymine with nonpolar analogues to evaluate the importance of hydrogen bonding in the polymerase active sites, while we used comparisons among a set of variably sized thymine analogues to measure the role of steric effects in the two enzymes. Steady-state kinetics measurements were carried out to evaluate activities for nucleotide insertion and extension. The results showed that both enzymes inserted nucleotides opposite nonpolar template bases with moderate to low efficiency, suggesting that both polymerases benefit from hydrogen bonding or other electrostatic effects involving the template base. Surprisingly, however, pol II inserted nonpolar nucleotide (dNTP) analogues into a primer strand with high (wild-type) efficiency, while pol IV handled them with an extremely low efficiency. Base pair extension studies showed that both enzymes bypass non-hydrogen-bonding template bases with moderately low efficiency, suggesting a possible beneficial role of minor groove hydrogen bonding interactions at the N-1 position. Measurement of the two polymerases' sensitivity to steric size changes showed that both enzymes were relatively flexible, yielding only small kinetic differences with increases or decreases in nucleotide size. Comparisons are made to recent data for DNA pol I (Klenow fragment), the archaeal polymerase Dpo4, and human pol kappa.
Figures

Similar articles
-
Varying DNA base-pair size in subangstrom increments: evidence for a loose, not large, active site in low-fidelity Dpo4 polymerase.Biochemistry. 2006 Mar 7;45(9):2772-8. doi: 10.1021/bi051961z. Biochemistry. 2006. PMID: 16503632
-
Mutagenicity and genotoxicity of (5'S)-8,5'-cyclo-2'-deoxyadenosine in Escherichia coli and replication of (5'S)-8,5'-cyclopurine-2'-deoxynucleosides in vitro by DNA polymerase IV, exo-free Klenow fragment, and Dpo4.Chem Res Toxicol. 2014 Feb 17;27(2):200-10. doi: 10.1021/tx4002786. Epub 2014 Jan 16. Chem Res Toxicol. 2014. PMID: 24392701 Free PMC article.
-
In vitro effects of a C4'-oxidized abasic site on DNA polymerases.Biochemistry. 2004 Mar 9;43(9):2656-63. doi: 10.1021/bi036028f. Biochemistry. 2004. PMID: 14992603
-
The steric hypothesis for DNA replication and fluorine hydrogen bonding revisited in light of structural data.Acc Chem Res. 2012 Aug 21;45(8):1237-46. doi: 10.1021/ar200303k. Epub 2012 Apr 23. Acc Chem Res. 2012. PMID: 22524491 Free PMC article. Review.
-
Escherichia coli Y family DNA polymerases.Front Biosci (Landmark Ed). 2011 Jun 1;16(8):3164-82. doi: 10.2741/3904. Front Biosci (Landmark Ed). 2011. PMID: 21622227 Review.
Cited by
-
DNA damage and interstrand cross-link formation upon irradiation of aryl iodide C-nucleotide analogues.J Org Chem. 2010 Feb 5;75(3):535-44. doi: 10.1021/jo902071y. J Org Chem. 2010. PMID: 20067226 Free PMC article.
-
Importance of steric effects on the efficiency and fidelity of transcription by T7 RNA polymerase.Biochemistry. 2011 Nov 29;50(47):10343-9. doi: 10.1021/bi2011465. Epub 2011 Nov 1. Biochemistry. 2011. PMID: 22044042 Free PMC article.
-
Conformational insights into the lesion and sequence effects for arylamine-induced translesion DNA synthesis: 19F NMR, surface plasmon resonance, and primer kinetic studies.Biochemistry. 2014 Jun 24;53(24):4059-71. doi: 10.1021/bi5003212. Epub 2014 Jun 10. Biochemistry. 2014. PMID: 24915610 Free PMC article.
References
-
- Latham GJ, McNees AG, De Corte B, Harris CM, Harris TM, O’Donnell M, Lloyd RS. Comparison of the efficiency of synthesis past single bulky DNA adducts in vivo and in vitro by the polymerase III holoenzyme. Chem Res Toxicol. 1996;9:1167–1175. - PubMed
-
- Rangarajan S, Woodgate R, Goodman MF. Replication restart in UV-irradiated Escherichia coli involving pols II, III, V, PriA, RecA and RecFOR proteins. Mol Microbiol. 2002;43:618–628. - PubMed
-
- Kobayashi S, Valentine MR, Pham P, O’Donnell M, Goodman MF. Fidelity of Escherichia coli DNA polymerase IV. J Biol Chem. 2002;277:34198–34207. - PubMed
-
- Wang Z, Lazarov E, O’Donnell M, Goodman MF. Resolving a fidelity paradox. J Biol Chem. 2002;277:4446–4454. - PubMed
-
- Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu Rev Biochem. 2002;71:17–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

