A sex-ratio meiotic drive system in Drosophila simulans. I: an autosomal suppressor
- PMID: 17988172
- PMCID: PMC2062475
- DOI: 10.1371/journal.pbio.0050292
A sex-ratio meiotic drive system in Drosophila simulans. I: an autosomal suppressor
Abstract
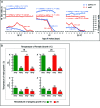
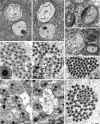
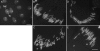
Sex ratio distortion (sex-ratio for short) has been reported in numerous species such as Drosophila, where distortion can readily be detected in experimental crosses, but the molecular mechanisms remain elusive. Here we characterize an autosomal sex-ratio suppressor from D. simulans that we designate as not much yang (nmy, polytene chromosome position 87F3). Nmy suppresses an X-linked sex-ratio distorter, contains a pair of near-perfect inverted repeats of 345 bp, and evidently originated through retrotransposition from the distorter itself. The suppression is likely mediated by sequence homology between the suppressor and distorter. The strength of sex-ratio is greatly enhanced by lower temperature. This temperature sensitivity was used to assign the sex-ratio etiology to the maturation process of the Y-bearing sperm, a hypothesis corroborated by both light microscope observations and ultrastructural studies. It has long been suggested that an X-linked sex-ratio distorter can evolve by exploiting loopholes in the meiotic machinery for its own transmission advantage, which may be offset by other changes in the genome that control the selfish distorter. Data obtained in this study help to understand this evolutionary mechanism in molecular detail and provide insight regarding its evolutionary impact on genomic architecture and speciation.
Conflict of interest statement
Figures


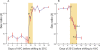
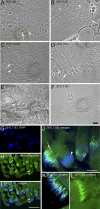
Comment in
-
Distorted sex ratios: a window into RNAi-mediated silencing.PLoS Biol. 2007 Nov 6;5(11):e303. doi: 10.1371/journal.pbio.0050303. PLoS Biol. 2007. PMID: 17988175 Free PMC article.
References
-
- Fisher RA. The genetical theory of natural selection. A complete variorium edition. Oxford: Oxford University Press; 1930. 318
-
- Edwards AWF (2000) Carl Düsing. on The Regulation of the Sex-Ratio . Theor Pop Biol. 1884;58:255–257. - PubMed
-
- Shaw RF, Mohler JD. The selective significance of the sex ratio. Am Nat. 1953;87:337–342.
-
- Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–488. - PubMed
-
- Bull JJ, Charnov EL. How fundamental are Fisherian sex ratios? In: Harvey PH, Partridge L, editors. Oxford surveys in evolutionary biology. New York: Oxford University Press; 1988. pp. 97–135.
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

