Dermal fibroblasts derived from fetal and postnatal humans exhibit distinct responses to insulin like growth factors
- PMID: 17988375
- PMCID: PMC2211318
- DOI: 10.1186/1471-213X-7-124
Dermal fibroblasts derived from fetal and postnatal humans exhibit distinct responses to insulin like growth factors
Abstract
Background: It has been well established that human fetuses will heal cutaneous wounds with perfect regeneration. Insulin-like growth factors are pro-fibrotic fibroblast mitogens that have important roles in both adult wound healing and during development, although their relative contribution towards fetal wound healing is currently unknown. We have compared responses to IGF-I and -II in human dermal fibroblast strains derived from early gestational age fetal (<14 weeks) and developmentally mature postnatal skin to identify any differences that might relate to their respective wound healing responses of regeneration or fibrosis.
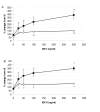
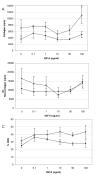
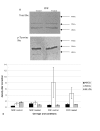
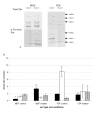
Results: We have established that the mitogenic response of fetal cells to both IGF-I and -II is much lower than that seen in postnatal dermal fibroblasts. Further, unlike postnatal cells, fetal cells fail to synthesise collagen in response to IGF-I, whereas they do increase synthesis in response to IGF-II. This apparent developmentally regulated difference in response to these related growth factors is also reflected in changes in the tyrosine phosphorylation pattern of a number of proteins. Postnatal cells exhibit a significant increase in phosphorylation of ERK 1 (p44) in response to IGF-I and conversely the p46 isoform of Shc on IGF-II stimulation. Fetal cells however only show a significant increase in an unidentified 100 kDa tyrosine-phosphorylated protein on stimulation with IGF-II.
Conclusion: Dermal fibroblasts exhibit different responses to the two forms of IGF depending on their developmental maturity. This may relate to the developmental transition in cutaneous wound healing from regeneration to fibrosis.
Figures





Similar articles
-
Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts.Endocrinology. 1997 Feb;138(2):741-50. doi: 10.1210/endo.138.2.4910. Endocrinology. 1997. PMID: 9003010
-
Differential gene expression in response to transforming growth factor-beta1 by fetal and postnatal dermal fibroblasts.Wound Repair Regen. 2007 Nov-Dec;15(6):897-906. doi: 10.1111/j.1524-475X.2007.00314.x. Wound Repair Regen. 2007. PMID: 18028139
-
Differential expression of receptor tyrosine kinases and Shc in fetal and adult rat fibroblasts: toward defining scarless versus scarring fibroblast phenotypes.Plast Reconstr Surg. 2000 Mar;105(3):972-9. doi: 10.1097/00006534-200003000-00021. Plast Reconstr Surg. 2000. PMID: 10724257
-
Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas.Mol Med. 2008 May-Jun;14(5-6):264-75. doi: 10.2119/2007-00101.Yu. Mol Med. 2008. PMID: 18231572 Free PMC article.
-
The immune function of dermal fibroblasts in skin defence against pathogens.Exp Dermatol. 2023 Sep;32(9):1326-1333. doi: 10.1111/exd.14858. Epub 2023 Jun 30. Exp Dermatol. 2023. PMID: 37387265 Review.
Cited by
-
Insulin-like Growth Factor-2 (IGF-2) in Fibrosis.Biomolecules. 2022 Oct 25;12(11):1557. doi: 10.3390/biom12111557. Biomolecules. 2022. PMID: 36358907 Free PMC article. Review.
-
Cutaneous scarring: a clinical review.Dermatol Res Pract. 2009;2009:625376. doi: 10.1155/2009/625376. Epub 2010 Feb 10. Dermatol Res Pract. 2009. PMID: 20585482 Free PMC article.
-
Bicomponent Cutaneous Cell Therapy for Early Burn Care: Manufacturing Homogeneity and Epidermis-Structuring Functions of Clinical Grade FE002-SK2 Allogeneic Dermal Progenitor Fibroblasts.Pharmaceutics. 2025 May 24;17(6):692. doi: 10.3390/pharmaceutics17060692. Pharmaceutics. 2025. PMID: 40574004 Free PMC article.
-
Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways.Am J Pathol. 2008 Jun;172(6):1580-90. doi: 10.2353/ajpath.2008.071021. Epub 2008 May 8. Am J Pathol. 2008. PMID: 18467708 Free PMC article.
-
Novel transcriptional profile in wrist muscles from cerebral palsy patients.BMC Med Genomics. 2009 Jul 14;2:44. doi: 10.1186/1755-8794-2-44. BMC Med Genomics. 2009. PMID: 19602279 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

