Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis
- PMID: 17988392
- PMCID: PMC2203987
- DOI: 10.1186/1465-9921-8-79
Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis
Abstract
Introduction: Short PLUNC1 (SPLUNC1) is the founding member of a family of proteins (PLUNCS) expressed in the upper respiratory tract and oral cavity, which may function in host defence. It is one of the most highly expressed genes in the upper airways and the protein has been detected in sputum and nasal secretions. The biology of the PLUNC family is poorly understood but in keeping with the putative function of the protein as an immune defence protein, a number of RNA and protein studies have indicated that SPLUNC1 is increased in inflammatory/infectious conditions such as Cystic Fibrosis (CF), COPD and allergic rhinitis.
Methods: We used immunohistochemistry to localise SPLUNC1 in lung tissue from patients with CF and a range of other lung diseases. We used a range of additional markers for distinct cell types to try to establish the exact site of secretion of SPLUNC1. We have complemented these studies with a molecular analysis of SPLUNC1 gene expression in primary human lung cell cultures and isolated inflammatory cell populations.
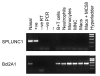
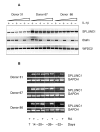
Results: In CF, expression of SPLUNC1 is significantly elevated in diseased airways and positive staining was noted in some of the inflammatory infiltrates. The epithelium of small airways of CF lung exhibit significantly increased SPLUNC1 staining compared to similar sized airways in non-CF lungs where staining is absent. Strong staining was also seen in mucous plugs in the airways, these included many inflammatory cells. No alveolar epithelial staining was noted in CF tissue. Airway epithelial staining did not co-localise with MUC5AC suggesting that the protein was not produced by goblet cells. Using serial sections stained with neutrophil elastase and CD68 we could not demonstrate co-localisation of SPLUNC1 with either neutrophils or macrophages/monocytes, indicating that these cells were not a source of SPLUNC1 in the airways of CF lungs. No change in staining pattern was noted in the small airways or lung parenchyma of other lung diseases studied including, COPD, emphysema or pneumonia where significant NE and CD68 staining was noted. Cultures of primary tracheobronchial epithelial cells were analysed by RT-PCR and showed that pro-inflammatory mediators did not induce expression of SPLUNC1. We have also shown that SPLUNC1 gene expression was not seen in isolated human mononuclear cells, macrophages or neutrophils.
Conclusion: These studies show that SPLUNC1 is specifically and significantly increased in the small airways of lungs from patients with CF. They further suggest that it is the airway epithelium that is responsible for the increased levels of SPLUNC1 in CF and not inflammatory cells; this could be a defensive response to the infectious component of the disease.
Figures


Similar articles
-
Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures.Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L990-L1001. doi: 10.1152/ajplung.00103.2013. Epub 2013 Oct 11. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24124190 Free PMC article.
-
SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration.Eur Respir J. 2018 Oct 4;52(4):1800668. doi: 10.1183/13993003.00668-2018. Print 2018 Oct. Eur Respir J. 2018. PMID: 30190268 Free PMC article.
-
SPLUNC1 (PLUNC) is expressed in glandular tissues of the respiratory tract and in lung tumours with a glandular phenotype.J Pathol. 2005 Mar;205(4):491-7. doi: 10.1002/path.1726. J Pathol. 2005. PMID: 15685591
-
Mammalian short palate lung and nasal epithelial clone 1 (SPLUNC1) in pH-dependent airway hydration.Int J Biochem Cell Biol. 2014 Jul;52:130-5. doi: 10.1016/j.biocel.2014.03.002. Epub 2014 Mar 13. Int J Biochem Cell Biol. 2014. PMID: 24631954 Free PMC article. Review.
-
Distribution of human PLUNC/BPI fold-containing (BPIF) proteins.Biochem Soc Trans. 2011 Aug;39(4):1023-7. doi: 10.1042/BST0391023. Biochem Soc Trans. 2011. PMID: 21787341 Review.
Cited by
-
Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways.FASEB J. 2012 Oct;26(10):4348-59. doi: 10.1096/fj.12-207431. Epub 2012 Jul 13. FASEB J. 2012. Retraction in: FASEB J. 2013 May;27(5):2081. doi: 10.1096/fj.12-207431RET. PMID: 22798424 Free PMC article. Retracted.
-
SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage.Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):11412-7. doi: 10.1073/pnas.0903609106. Epub 2009 Jun 18. Proc Natl Acad Sci U S A. 2009. PMID: 19541605 Free PMC article.
-
Immunogenic senescence sensitizes lung cancer to LUNX-targeting therapy.Cancer Immunol Immunother. 2022 Jun;71(6):1403-1417. doi: 10.1007/s00262-021-03077-1. Epub 2021 Oct 21. Cancer Immunol Immunother. 2022. PMID: 34674012 Free PMC article.
-
CC16 drives VLA-2-dependent SPLUNC1 expression.Front Immunol. 2023 Nov 20;14:1277582. doi: 10.3389/fimmu.2023.1277582. eCollection 2023. Front Immunol. 2023. PMID: 38053993 Free PMC article.
-
Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures.Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L990-L1001. doi: 10.1152/ajplung.00103.2013. Epub 2013 Oct 11. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24124190 Free PMC article.
References
-
- Bingle CD, Bingle L. Characterisation of the human plunc gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochim Biophys Acta. 2000;1493:363–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

