Drug monitoring: simultaneous analysis of lamotrigine, oxcarbazepine, 10-hydroxycarbazepine, and zonisamide by HPLC-UV and a rapid GC method using a nitrogen-phosphorus detector for levetiracetam
- PMID: 17988451
- PMCID: PMC2231334
- DOI: 10.1093/chromsci/45.9.616
Drug monitoring: simultaneous analysis of lamotrigine, oxcarbazepine, 10-hydroxycarbazepine, and zonisamide by HPLC-UV and a rapid GC method using a nitrogen-phosphorus detector for levetiracetam
Abstract
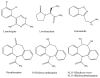
A high-performance liquid chromatography (HPLC) assay using UV detection is described for the simultaneous measurement of the newer generation anti-epileptic medications lamotrigine, oxcarbazepine (parent drug and active metabolite 10- hydroxycarbazepine), and zonisamide. Detection of all four compounds can be done at 230 nm; however, there is a potential interference with zonisamide in patients on clonazepam therapy. Therefore, the method uses dual wavelength detection: 230 nm for oxcarbazepine and 10-hydroxycarbazepine and 270 nm for lamotrigine and zonisamide. In addition, a simple gas chromatography method using a nitrogen-phosphorus detector is described for the measurement of levetiracetam, another of the recently approved anti-epileptic medications. For both methods, limits of quantitation, linearities, accuracies, and imprecisions cover the therapeutic range for drug monitoring of patients. A wide variety of clinical drugs, including other anti-epileptic drugs, do not interfere with these assays. These procedures would be of special interest to clinical laboratories, particularly due to the limited availability of immunoassays for newer generation anti-epileptic medications and that therapeutic uses of these drugs are expanding beyond epilepsy to other neurologic and psychiatric disorders.
Figures

Similar articles
-
Simultaneous Quantitation of Lamotrigine, Levetiracetam, 10-Hydroxycarbazepine, Topiramate, and Zonisamide in Serum Using HPLC-MS/MS.Methods Mol Biol. 2016;1383:29-37. doi: 10.1007/978-1-4939-3252-8_4. Methods Mol Biol. 2016. PMID: 26660171
-
Simultaneous determination of lamotrigine, zonisamide, and carbamazepine in human plasma by high-performance liquid chromatography.Biomed Chromatogr. 2007 Mar;21(3):225-8. doi: 10.1002/bmc.753. Biomed Chromatogr. 2007. PMID: 17230449 Free PMC article.
-
Drug monitoring and toxicology: a procedure for the monitoring of levetiracetam and zonisamide by HPLC-UV.J Anal Toxicol. 2006 Jan-Feb;30(1):27-30. doi: 10.1093/jat/30.1.27. J Anal Toxicol. 2006. PMID: 16620528
-
Oxcarbazepine, topiramate, zonisamide, and levetiracetam: potential use in neuropathic pain.Am J Geriatr Pharmacother. 2003 Sep;1(1):18-37. doi: 10.1016/s1543-5946(03)80013-2. Am J Geriatr Pharmacother. 2003. PMID: 15555463 Review.
-
Levetiracetam, oxcarbazepine, remacemide and zonisamide for drug resistant localization-related epilepsy: a systematic review.Epilepsy Res. 2001 Sep;46(3):259-70. doi: 10.1016/s0920-1211(01)00287-x. Epilepsy Res. 2001. PMID: 11518627
Cited by
-
Radiolabeling of Zonisamide for a Diagnostic Perspective.Curr Radiopharm. 2024;17(1):91-98. doi: 10.2174/0118744710249156231002115024. Curr Radiopharm. 2024. PMID: 37818565
-
Determination and Validation of Zonisamide and its Four Related Substances by HPLC and UV-Spectrophotometry.Indian J Pharm Sci. 2010 May;72(3):302-6. doi: 10.4103/0250-474X.70474. Indian J Pharm Sci. 2010. PMID: 21188037 Free PMC article.
-
Therapeutic Drug Monitoring of the Newer Anti-Epilepsy Medications.Pharmaceuticals (Basel). 2010 Jun 11;3(6):1909-1935. doi: 10.3390/ph3061909. Pharmaceuticals (Basel). 2010. PMID: 20640233 Free PMC article.
-
Determination of lamotrigine in human plasma by HPLC-PDA. Application to forensic samples.Forensic Sci Med Pathol. 2025 Mar;21(1):1-10. doi: 10.1007/s12024-024-00812-9. Epub 2024 Apr 10. Forensic Sci Med Pathol. 2025. PMID: 38598081 Free PMC article.
-
Determination of Levetiracetam in Human Plasma by Dispersive Liquid-Liquid Microextraction Followed by Gas Chromatography-Mass Spectrometry.J Anal Methods Chem. 2016;2016:5976324. doi: 10.1155/2016/5976324. Epub 2016 Oct 17. J Anal Methods Chem. 2016. PMID: 27830105 Free PMC article.
References
-
- LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. J Am Med Assoc. 2004;291(5):605–14. - PubMed
-
- LaRoche SM, Helmers SL. The new antiepileptic drugs: clinical applications. J Am Med Assoc. 2004;291(5):615–20. - PubMed
-
- Bialer M. The pharmacokinetics and interactions of new antiepileptic drugs: an overview. Ther Drug Monit. 2005;27(6):722–26. - PubMed
-
- Fraser AD, MacNeil W, Isner AF, Camfield PR. Lamotrigine analysis in serum by high-performance liquid chromatography. Ther Drug Monit. 1995;17(2):174–78. - PubMed
-
- Lensmeyer GL, Gidal BE, Wiebe DA. Optimized high-performance liquid chromatographic method for determination of lamotrigine in serum with concomitant determination of phenytoin, carbamazepine, and carbamazepine epoxide. Ther Drug Monit. 1997;19(3):292–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

