Two Gr genes underlie sugar reception in Drosophila
- PMID: 17988633
- PMCID: PMC2096712
- DOI: 10.1016/j.neuron.2007.10.024
Two Gr genes underlie sugar reception in Drosophila
Abstract
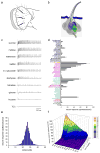
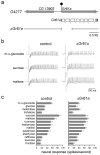
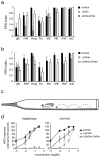
We have analyzed the molecular basis of sugar reception in Drosophila. We define the response spectrum, concentration dependence, and temporal dynamics of sugar-sensing neurons. Using in situ hybridization and reporter gene expression, we identify members of the Gr5a-related taste receptor subfamily that are coexpressed in sugar neurons. Neurons expressing reporters of different Gr5a-related genes send overlapping but distinct projections to the brain and thoracic ganglia. Genetic analysis of receptor genes shows that Gr5a is required for response to one subset of sugars and Gr64a for response to a complementary subset. A Gr5a;Gr64a double mutant shows no physiological or behavioral responses to any tested sugar. The simplest interpretation of our results is that Gr5a and Gr64a are each capable of functioning independently of each other within individual sugar neurons and that they are the primary receptors used in the labellum to detect sugars.
Figures




References
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Cheng E, Chang C-M, Sun HY. An improved proboscis extension assay. Drosophila. Information Newsletter. 1992;6
-
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

