Processing of tactile information by the hippocampus
- PMID: 17989221
- PMCID: PMC2084335
- DOI: 10.1073/pnas.0708611104
Processing of tactile information by the hippocampus
Abstract
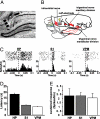
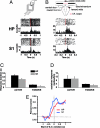
The ability to detect unusual events occurring in the environment is essential for survival. Several studies have pointed to the hippocampus as a key brain structure in novelty detection, a claim substantiated by its wide access to sensory information through the entorhinal cortex and also distinct aspects of its intrinsic circuitry. Novelty detection is implemented by an associative match-mismatch algorithm involving the CA1 and CA3 hippocampal subfields that compares the stream of sensory inputs received by CA1 to the stored representation of spatiotemporal sequences in CA3. In some rodents, including the rat, the highly sensitive facial whiskers are responsible for providing accurate tactile information about nearby objects. Surprisingly, however, not much is known about how inputs from the whiskers reach CA1 and how they are processed therein. Using concurrent multielectrode neuronal recordings and chemical inactivation in behaving rats, we show that trigeminal inputs from the whiskers reach the CA1 region through thalamic and cortical relays associated with discriminative touch. Ensembles of hippocampal neurons also carry precise information about stimulus identity when recorded during performance in an aperture-discrimination task using the whiskers. We also found broad similarities between tactile responses of trigeminal stations and the hippocampus during different vigilance states (wake and sleep). Taken together, our results show that tactile information associated with fine whisker discrimination is readily available to the hippocampus for dynamic updating of spatial maps.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

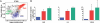
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

