Temporal and spatial activation of caspase-like enzymes induced by self-incompatibility in Papaver pollen
- PMID: 17989229
- PMCID: PMC2084342
- DOI: 10.1073/pnas.0705826104
Temporal and spatial activation of caspase-like enzymes induced by self-incompatibility in Papaver pollen
Abstract
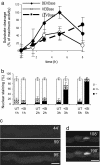
Caspase-like proteases are universal mediators of programmed cell death (PCD). Because plants have no caspase homologs, establishing the nature of their caspase-like activities is of considerable importance to our understanding of PCD in plants. Caspase-3, displaying DEVD specificity, is a key executioner caspase in animal cells. Self-incompatibility (SI) is an important mechanism to prevent self-fertilization and inbreeding in higher plants by inhibiting incompatible pollen. In Papaver rhoeas, SI activates a caspase-3-like/DEVDase activity in incompatible pollen that plays a pivotal role in regulating PCD. Here we characterize the SI-induced caspase-like activities in detail; our work provides insights into the temporal and spatial activation of plant caspase-like enzymes. We show that SI also activates a VEIDase and a LEVDase and that the VEIDase plays a role in SI-induced PCD. The DEVDase and VEIDase are activated remarkably rapidly: detectable within 1-2 h after SI induction; the LEVDase activity peaks later. Importantly, we show live-cell imaging of a DEVDase activity in a higher plant cell; the SI-activated DEVDase has a cytosolic and nuclear localization. We also demonstrate that SI induces a rapid and substantial cytosolic acidification that matches the in vitro pH optima for the SI-induced caspase activities. Because both cytosolic acidification and nuclear caspase localization are observed during apoptosis in animal cells, our data provide striking parallels between SI-induced PCD and apoptosis in animal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


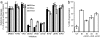

References
-
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, et al. J Biol Chem. 1997;272:17907–17911. - PubMed
-
- Uren GA, O'Rourke K, Pisabarro TM, Seshgari S, Koonin VE, Dixit VM. Mol Cell. 2000;6:961–967. - PubMed
-
- Woltering EJ. Trends Plants Sci. 2004;9:469–472. - PubMed
-
- Madeo F, Herker E, Maldener C, Wissing S, Lachelt S, Herian M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Frohlich KU. Mol Cell. 2002;9:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

