Human motor corpus callosum: topography, somatotopy, and link between microstructure and function
- PMID: 17989279
- PMCID: PMC6673264
- DOI: 10.1523/JNEUROSCI.2320-07.2007
Human motor corpus callosum: topography, somatotopy, and link between microstructure and function
Abstract
The corpus callosum (CC) is the principal white matter fiber bundle connecting neocortical areas of the two hemispheres. Although an object of extensive research, important details about the anatomical and functional organization of the human CC are still largely unknown. Here we focused on the callosal motor fibers (CMFs) that connect the primary motor cortices (M1) of the two hemispheres. Topography and somatotopy of CMFs were explored by using a combined functional magnetic resonance imaging/diffusion tensor imaging fiber-tracking procedure. CMF microstructure was assessed by fractional anisotropy (FA), and CMF functional connectivity between the hand areas of M1 was measured by interhemispheric inhibition using paired-pulse transcranial magnetic stimulation. CMFs mapped onto the posterior body and isthmus of the CC, with hand CMFs running significantly more anteriorly and ventrally than foot CMFs. FA of the hand CMFs but not FA of the foot CMFs correlated linearly with interhemispheric inhibition between the M1 hand areas. Findings demonstrate that CMFs connecting defined body representations of M1 map onto a circumscribed region in the CC in a somatotopically organized manner. The significant and topographically specific positive correlation between FA and interhemispheric inhibition strongly suggests that microstructure can be directly linked to functional connectivity. This provides a novel way of exploring human brain function that may allow prediction of functional connectivity from variability of microstructure in healthy individuals, and potentially, abnormality of functional connectivity in neurological or psychiatric patients.
Figures
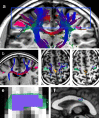
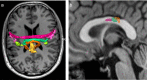

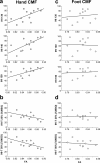
Comment in
-
Anatomy of the corpus callosum reveals its function.J Neurosci. 2008 Feb 13;28(7):1535-6. doi: 10.1523/JNEUROSCI.5426-07.2008. J Neurosci. 2008. PMID: 18272674 Free PMC article. No abstract available.
References
-
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. - PubMed
-
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain. 1999;122:855–870. - PubMed
-
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. - PubMed
-
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
