The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus
- PMID: 17989282
- PMCID: PMC3363962
- DOI: 10.1523/JNEUROSCI.1898-07.2007
The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus
Abstract
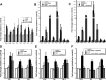
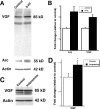
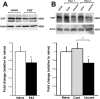
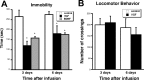
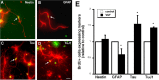
Brain-derived neurotrophic factor (BDNF) is upregulated in the hippocampus by antidepressant treatments, and BDNF produces antidepressant-like effects in behavioral models of depression. In our previous work, we identified genes induced by BDNF and defined their specific roles in hippocampal neuronal development and plasticity. To identify genes downstream of BDNF that may play roles in psychiatric disorders, we examined a subset of BDNF-induced genes also regulated by 5-HT (serotonin), which includes the neuropeptide VGF (nonacronymic). To explore the function of VGF in depression, we first investigated the expression of the neuropeptide in animal models of depression. VGF was downregulated in the hippocampus after both the learned helplessness and forced swim test (FST) paradigms. Conversely, VGF infusion in the hippocampus of mice subjected to FST reduced the time spent immobile for up to 6 d, thus demonstrating a novel role for VGF as an antidepressant-like agent. Recent evidence indicates that chronic treatment of rodents with antidepressants increases neurogenesis in the adult dentate gyrus and that neurogenesis is required for the behavioral effects of antidepressants. Our studies using [(3)H]thymidine and bromodeoxyuridine as markers of DNA synthesis indicate that chronic VGF treatment enhances proliferation of hippocampal progenitor cells both in vitro and in vivo with survival up to 21 d. By double immunocytochemical analysis of hippocampal neurons, we demonstrate that VGF increases the number of dividing cells that express neuronal markers in vitro. Thus, VGF may act downstream of BDNF and exert its effects as an antidepressant-like agent by enhancing neurogenesis in the hippocampus.
Figures



References
-
- Adell A, Castro E, Celada P, Bortolozzi A, Pazos A, Artigas F. Strategies for producing faster acting antidepressants. Drug Discov Today. 2005;10:578–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
