Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin
- PMID: 17989306
- PMCID: PMC6673250
- DOI: 10.1523/JNEUROSCI.0719-07.2007
Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin
Abstract
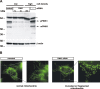
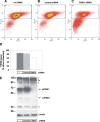
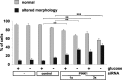
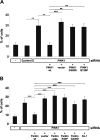
Degeneration of dopaminergic neurons in the substantia nigra is characteristic for Parkinson's disease (PD), the second most common neurodegenerative disorder. Mitochondrial dysfunction is believed to contribute to the etiology of PD. Although most cases are sporadic, recent evidence points to a number of genes involved in familial variants of PD. Among them, a loss-of-function of phosphatase and tensin homolog-induced kinase 1 (PINK1; PARK6) is associated with rare cases of autosomal recessive parkinsonism. In HeLa cells, RNA interference-mediated downregulation of PINK1 results in abnormal mitochondrial morphology and altered membrane potential. Morphological changes of mitochondria can be rescued by expression of wild-type PINK1 but not by PD-associated PINK1 mutants. Moreover, primary cells derived from patients with two different PINK1 mutants showed a similar defect in mitochondrial morphology. Human parkin but not PD-associated mutants could rescue mitochondrial pathology in human cells like wild-type PINK1. Our results may therefore suggest that PINK1 deficiency in humans results in mitochondrial abnormalities associated with cellular stress, a pathological phenotype, which can be ameliorated by enhanced expression of parkin.
Figures


References
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. - PubMed
-
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
