Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin
- PMID: 17989779
- PMCID: PMC2041817
- DOI: 10.1371/journal.pntd.0000002
Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin
Abstract
Background: Buruli ulcer caused by Mycobacterium ulcerans is an infection of the subcutaneous tissue leading to chronic necrotising skin ulcers. The pathogenesis is associated with the cytocidal and immunosuppressive activities of a macrolide toxin. Histopathological hallmark of progressing disease is a poor inflammatory response despite of clusters of extracellular bacilli. While traditionally wide excision of the infected tissue was the standard treatment, provisional WHO guidelines now recommend an eight week pre-treatment with streptomycin and rifampicin.
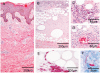
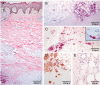
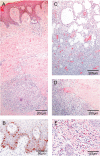
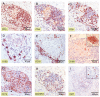
Methodology/principal findings: We conducted a detailed immunohistochemical analysis of tissue samples from Buruli patients who received antibiotic treatment. Cellular immune response along with bacterial load and distribution were monitored. We demonstrate that this treatment leads to the development of highly organized cellular infiltration surrounding areas of coagulative necrosis. Diffuse infiltrates, granulomas and dense lymphocyte aggregation close to vessels were observed. Mycobacterial material was primarily located inside mononuclear phagocytes and microcolonies consisting of extracellular rod-shaped mycobacteria were no longer found. In observational studies some patients showed no clinical response to antibiotic treatment. Corresponding to that, one of five lesions analysed presented with huge clusters of rod-shaped bacilli but no signs of infiltration.
Conclusions/significance: Results signify that eight weeks of antibiotic treatment reverses local immunosuppression and leads to an active inflammatory process in different compartments of the skin. Structured leukocyte infiltrates with unique signatures indicative for healing processes developed at the margins of the lesions. It remains to be analysed whether antibiotic resistance of certain strains of M. ulcerans, lacking patient compliance or poor drug quality are responsible for the absent clinical responses in some patients. In future, analysis of local immune responses could serve as a suitable surrogate marker for the efficacy of alternative treatment strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Asiedu A, Scherpbier RW, Raviglione M. Geneva, Switzerland: World Health Organization; 2000. Buruli ulcer, Mycobacterium ulcer infection.
-
- George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–857. - PubMed
-
- Snyder D, Small PL. Uptake and cellular actions of mycolactone, a virulence determinant for Mycobacterium ulcerans. Microb Pathog. 2003;34:91–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

