Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43
- PMID: 17990308
- PMCID: PMC2636557
- DOI: 10.1002/glia.20598
Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43
Abstract
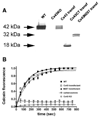
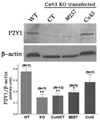
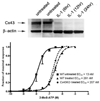
Gap junction proteins, connexins, provide intercellular channels that allow ions and small signaling molecules to be transmitted to adjacent coupled cells. Besides this function, it is becoming apparent that connexins also exert channel-independent effects, which are likely mediated by processes involving protein-protein interactions. Although a number of connexin interacting proteins have been identified, only little is known about the functional consequences of such interactions. We have previously shown that deletion of the astrocytic gap junction protein, connexin43 (Cx43) causes a right-ward shift in the dose-response curve to P2Y1R agonists and decreased P2Y1R expression levels. To evaluate whether these changes were due to reduced gap junctional communication or to protein-protein interactions, Cx43-null astrocytes were transfected with full-length Cx43 and Cx43 domains, and P2Y1R function and expression levels evaluated. Results indicate that restoration of P2Y1R function is independent of gap junctional communication and that the Cx43 carboxyl terminus spanning the SH3 binding domain (260-280) participates in the rescue of P2Y1R pharmacological behavior (shifting to the left the P2Y1R dose-response curve) without affecting its expression levels. These results suggest that the Cx43 carboxyl-terminus domain provides a binding site for an intracellular molecule, most likely a member of the c-Src tyrosine kinase family, which affects P2Y1R-induced calcium mobilization. It is here proposed that a nonchannel function of Cx43 is to serve as a decoy for such kinases. Such modulation of P2Y1R is expected to influence several neural cell functions, especially under inflammation and neurodegenerative disorders where expression levels of Cx43 are decreased.
Copyright (c) 2007 Wiley-Liss, Inc.
Figures


References
-
- Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D’Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7:2533–2537. - PubMed
-
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

