Differential expression of gamma-aminobutyric acid receptor A (GABA(A)) and effects of homocysteine
- PMID: 17990949
- PMCID: PMC3182488
- DOI: 10.1515/CCLM.2007.342
Differential expression of gamma-aminobutyric acid receptor A (GABA(A)) and effects of homocysteine
Abstract
Background: gamma-Aminobutyric acid (GABA) is a known inhibitory neurotransmitter in the mammalian central nervous system, and homocysteine (Hcy) behaves as an antagonist for GABA(A) receptor. Although the properties and functions of GABA(A) receptors are well studied in mouse neural tissue, its presence and significance in non-neural tissue remains obscure. The aim of the present study was to examine the expression of GABA(A) receptor and its subunits in non-neural tissue.
Methods: The mice were analyzed. The presence of GABA(A) receptor and its subunits was evaluated using Western blot and reverse transcription polymerase chain reaction.
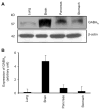
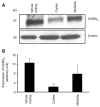
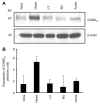
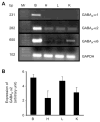
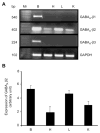
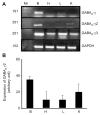
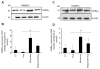
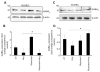
Results: We report that GABA(A) receptor protein is abundant in the renal medulla, cortex, heart, left ventricle, aorta and pancreas. Low levels of GABA(A) receptor protein were detected in the atria of the heart, right ventricle, lung and stomach. The mRNA protein expression of GABA(A) receptor subunit shows that alpha1, beta1, beta3 and gamma1 subunits are present only in brain. The mRNA protein expression levels of GABA(A) receptor alpha2, alpha6, beta2 and gamma3 subunits were highly expressed in brain compared to other tested tissue, while GABA(A) receptor gamma2 subunit was expressed only in brain and kidney. Treatment of microvascular endothelial cells with Hcy decreased GABA(A) receptor protein level, which was restored to its baseline level in the presence of GABA(A) receptor agonist, muscimol. The distribution of GABA(A) and GABA(B) receptors in wild type mice was determined and tissue-specific expression patterns were found showing that several receptor subtypes were also expressed in the central nervous system.
Conclusions: Hcy, a GABA(A) agonist, was found to decrease GABA(A) expression levels. These data enlarge knowledge on distribution of GABA receptors and give novel ideas of the effects of Hcy on different organs.
Figures
References
-
- Hunt MJ, Tyagi SC. Peroxisome proliferators compete and ameliorate Hcy-mediated endocardial endothelial cell activation. Am J Physiol Cell Physiol. 2002;283:C1073–9. - PubMed
-
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989;10:401–7. - PubMed
-
- Martin IL, Dunn SM. GABA receptors. Tocris Review. Tocris Cookson Ltd; 2002. [Accessed 2002]. www.biotrend.com/download/gabarev.pdf.
-
- Awapara J, Landua AJ, Fuerst R, Seale B. Free gamma-aminobutyric acid in brain. J Biol Chem. 1950;87:35–9. - PubMed
-
- Roberts E, Frankel S. Gamma-aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
