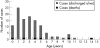Severe complications of chickenpox in hospitalised children in the UK and Ireland
- PMID: 17991685
- PMCID: PMC2066097
- DOI: 10.1136/adc.2007.123232
Severe complications of chickenpox in hospitalised children in the UK and Ireland
Abstract
Aims: To estimate the annual incidence of hospitalisations due to severe complications of varicella, describe the complications and estimate annual mortality.
Methods: Active surveillance throughout the UK and Ireland for 13 months by paediatricians notifying cases to the British Paediatric Surveillance Unit and completing a questionnaire. The case definition was any child aged <16 years hospitalised with complicated varicella, as defined by a list of conditions, or admitted to ICU/HDU with varicella.
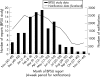
Results: 188 cases were notified for the surveillance period, of which 112 (0.82/100 000 children/year) met the case definition and were not duplicates. Confirmed cases had a median age of 3 years (range 0-14). The complications were: bacteraemia/septic shock (n = 30), pneumonia (n = 30), encephalitis (n = 26), ataxia (n = 25), toxic shock syndrome/toxin-mediated disease (n = 14), necrotising fasciitis (n = 7), purpura fulminans/disseminated coagulopathy (n = 5), fulminant varicella (n = 5) and neonatal varicella (n = 3). 52 children (46%) had additional bacterial infections. Six deaths were due, or possibly due, to varicella, including one intrauterine death. Four of the other five children who died (ages 2-14 years) had a pre-existing medical condition. Sequelae on discharge were reported for 41 cases (40%), most frequently ataxia or skin scarring. The median length of hospital stay was 7 days (range 1-68).
Conclusions: This study provides a minimum estimate of severe complications and death resulting from varicella in children in the UK and Ireland. Most complications, excluding deaths, occur in otherwise healthy children and thus would be preventable only through a universal childhood immunisation programme.
Conflict of interest statement
Competing interests: Health Protection Scotland has received funding for research and conference attendance from pharmaceutical companies. RB and AF are occasionally supported by pharmaceutical companies to attend or present at scientific meetings; any fees offered are directed to a university research account. PTH has received funds from vaccine manufacturers to attend conferences and meetings, and St. George's, University of London has received research grants from vaccine manufacturers on behalf of PTH.
Comment in
-
Should the UK introduce varicella vaccine?Arch Dis Child. 2007 Dec;92(12):1051-2. doi: 10.1136/adc.2007.130518. Epub 2007 Nov 8. Arch Dis Child. 2007. PMID: 17991684 Free PMC article. Review.
References
-
- Gershon A A, Takahashi M, Seward J. Varicella vaccine. In: Plotkin SA, Orenstein WA, eds. Vaccines. 4th ed. Philadelphia: WB Saunders, 2004783–823.
-
- Bramley J C, Jones I G. Epidemiology of chickenpox in Scotland: 1981 to 1998. Commun Dis Public Health 20003(4)282–287. - PubMed
-
- Brisson M, Edmunds W J. Epidemiology of varicella‐zoster virus in England and Wales. J Med Virol 200370(Suppl 1)S9–14. - PubMed
-
- Ross A M, Fleming D M. Chickenpox increasingly affects preschool children. Commun Dis Public Health 20003(3)213–215. - PubMed
-
- Salisbury D, Ramsay M, Noakes K. eds. Immunisation against infectious disease. 3rd ed. London: Stationary Office 2006
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous