Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: the neurosteroid opioid connection
- PMID: 17991694
- PMCID: PMC2375576
- DOI: 10.1113/jphysiol.2007.146233
Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: the neurosteroid opioid connection
Abstract
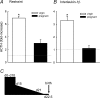
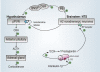
In late pregnancy maternal hypothalamo-pituitary-adrenal (HPA) axis responses to emotional and physical stressors are attenuated. This is expected to minimize the detrimental programming effects of glucocorticoid exposure on the fetuses. We have utilized a model of immune challenge, systemic administration of interleukin-1beta (IL-1beta), to investigate the underlying mechanisms. Intravenous IL-1beta activates corticotropin-releasing hormone (CRH) neurones in the parvocellular division of the paraventricular nucleus (pPVN) via noradrenergic (A2 cell group) neurones in the nucleus tractus solitarii (NTS). Despite comparable activation of these brainstem neurones by IL-1beta in virgin and in late pregnant rats, pPVN CRH neurones are activated only in virgin rats. As a consequence IL-1beta fails to evoke ACTH and corticosterone secretion in late pregnant rats, in contrast to virgin rats. Suppressed responsiveness of the CRH neurones, and hence the HPA axis, following IL-1beta in late pregnancy is explained by presynaptic inhibition of noradrenaline release in the pPVN, due to increased endogenous enkephalin and mu-opioid receptor production in brainstem NTS neurones. The factor that signals to the brain the pregnancy status of the animal and stimulates opioid production in the brainstem is allopregnanolone, a neurosteroid metabolite of progesterone. The supporting evidence for these mechanisms is discussed.
Figures

Similar articles
-
Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone.J Neurosci. 2009 May 20;29(20):6449-60. doi: 10.1523/JNEUROSCI.0708-09.2009. J Neurosci. 2009. PMID: 19458216 Free PMC article.
-
Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat.Stress. 2011 Jan;14(1):6-12. doi: 10.3109/10253890.2010.482628. Epub 2010 Jul 28. Stress. 2011. PMID: 20666638 Review.
-
Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats.J Neurosci. 2005 May 25;25(21):5117-26. doi: 10.1523/JNEUROSCI.0866-05.2005. J Neurosci. 2005. PMID: 15917452 Free PMC article.
-
Reduced hypothalamo-pituitary-adrenal axis stress responses in late pregnancy: central opioid inhibition and noradrenergic mechanisms.Ann N Y Acad Sci. 2008 Dec;1148:428-38. doi: 10.1196/annals.1410.032. Ann N Y Acad Sci. 2008. PMID: 19120138 Review.
-
5α-Reduced neurosteroids sex-dependently reverse central prenatal programming of neuroendocrine stress responses in rats.J Neurosci. 2015 Jan 14;35(2):666-77. doi: 10.1523/JNEUROSCI.5104-13.2015. J Neurosci. 2015. PMID: 25589761 Free PMC article.
Cited by
-
A Novel, Synthetic, Neuroactive Steroid Is Effective at Decreasing Depression-Like Behaviors and Improving Maternal Care in Preclinical Models of Postpartum Depression.Front Endocrinol (Lausanne). 2018 Nov 23;9:703. doi: 10.3389/fendo.2018.00703. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30532739 Free PMC article.
-
Prenatal resident-intruder stress decreases levels of allopregnanolone in the cortex, hypothalamus, and midbrain of males, and increases levels in the hippocampus and cerebellum of female, juvenile rat offspring.Neurobiol Stress. 2020 Mar 3;12:100214. doi: 10.1016/j.ynstr.2020.100214. eCollection 2020 May. Neurobiol Stress. 2020. PMID: 32258257 Free PMC article.
-
The Allopregnanolone Response to Acute Stress in Females: Preclinical and Clinical Studies.Biomolecules. 2022 Sep 8;12(9):1262. doi: 10.3390/biom12091262. Biomolecules. 2022. PMID: 36139100 Free PMC article. Review.
-
Fentanyl-induced respiratory depression is attenuated in pregnant patients.Drug Des Devel Ther. 2017 Nov 22;11:3325-3332. doi: 10.2147/DDDT.S147304. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29200828 Free PMC article. Clinical Trial.
-
Suppression of the febrile response in late gestation: evidence, mechanisms and outcomes.J Neuroendocrinol. 2008 Apr;20(4):508-14. doi: 10.1111/j.1365-2826.2008.01666.x. Epub 2008 Feb 8. J Neuroendocrinol. 2008. PMID: 18266941 Free PMC article. Review.
References
-
- Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. - PubMed
-
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. - PubMed
-
- Benediktsson R, Calder AA, Edwards CRW, Seckl JR. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol. 1997;46:161–166. - PubMed
-
- Besedovsky HO, Del Rey A, Klusman I, Furukawa H, Monge Arditi G, Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40:613–618. - PubMed
-
- Brunton PJ, Bales J, Russell JA. Neuroendocrine stress but not feeding responses to centrally administered neuropeptide Y are suppressed in pregnant rats. Endocrinology. 2006;147:3737–3745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

