Expression microarray analysis implicates apoptosis and interferon-responsive mechanisms in susceptibility to experimental cerebral malaria
- PMID: 17991715
- PMCID: PMC2111112
- DOI: 10.2353/ajpath.2007.070630
Expression microarray analysis implicates apoptosis and interferon-responsive mechanisms in susceptibility to experimental cerebral malaria
Abstract
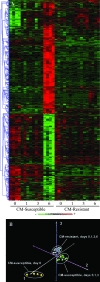
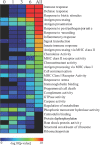
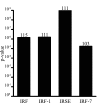
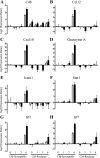
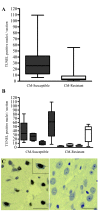
Specific local brain responses, influenced by parasite sequestration and host immune system activation, have been implicated in the development of cerebral malaria. This study assessed whole-brain transcriptional responses over the course of experimental cerebral malaria by comparing genetically resistant and susceptible inbred mouse strains infected with Plasmodium berghei ANKA. Computational methods were used to identify differential patterns of gene expression. Overall, genes that showed the most transcriptional activity were differentially expressed in susceptible mice 1 to 2 days before the onset of characteristic symptoms of cerebral malaria. Most of the differentially expressed genes identified were associated with immune-related gene ontology categories. Further analysis to identify interaction networks and to examine patterns of transcriptional regulation within the set of identified genes implicated a central role for both interferon-regulated processes and apoptosis in the pathogenesis of cerebral malaria. Biological relevance of these genes and pathways was confirmed using quantitative RT-PCR and histopathological examination of the brain for apoptosis. The application of computational biology tools to examine systematically the disease progression in cerebral malaria can identify important transcriptional programs activated during its pathogenesis and may serve as a methodological approach to identify novel targets for therapeutic intervention.
Figures
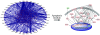
Comment in
-
Cerebral malaria pathogenesis: what can we learn from microarray analysis?Am J Pathol. 2007 Dec;171(6):1729-32. doi: 10.2353/ajpath.2007.070917. Epub 2007 Nov 8. Am J Pathol. 2007. PMID: 17991710 Free PMC article. No abstract available.
Similar articles
-
Gene expression analysis reveals early changes in several molecular pathways in cerebral malaria-susceptible mice versus cerebral malaria-resistant mice.BMC Genomics. 2007 Dec 6;8:452. doi: 10.1186/1471-2164-8-452. BMC Genomics. 2007. PMID: 18062806 Free PMC article.
-
Simultaneous host and parasite expression profiling identifies tissue-specific transcriptional programs associated with susceptibility or resistance to experimental cerebral malaria.BMC Genomics. 2006 Nov 22;7:295. doi: 10.1186/1471-2164-7-295. BMC Genomics. 2006. PMID: 17118208 Free PMC article.
-
Parasite burden and CD36-mediated sequestration are determinants of acute lung injury in an experimental malaria model.PLoS Pathog. 2008 May 16;4(5):e1000068. doi: 10.1371/journal.ppat.1000068. PLoS Pathog. 2008. PMID: 18483551 Free PMC article.
-
Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei.Mamm Genome. 2018 Aug;29(7-8):488-506. doi: 10.1007/s00335-018-9752-9. Epub 2018 Jun 19. Mamm Genome. 2018. PMID: 29922917 Review.
-
Changes in brain metabolites in experimental cerebral malaria infection with plasmodium berghei ANKA: a literature review.J Pak Med Assoc. 2014 Oct;64(10):1179-85. J Pak Med Assoc. 2014. PMID: 25823161 Review.
Cited by
-
Disease and phenotype gene set analysis of disease-based gene expression in mouse and human.Physiol Genomics. 2010 Oct;42A(2):162-7. doi: 10.1152/physiolgenomics.00008.2010. Epub 2010 Aug 3. Physiol Genomics. 2010. PMID: 20682848 Free PMC article.
-
Pathway-GPS and SIGORA: identifying relevant pathways based on the over-representation of their gene-pair signatures.PeerJ. 2013 Dec 19;1:e229. doi: 10.7717/peerj.229. eCollection 2013 Dec 19. PeerJ. 2013. PMID: 24432194 Free PMC article.
-
Cerebral malaria in children is associated with long-term cognitive impairment.Pediatrics. 2008 Jul;122(1):e92-9. doi: 10.1542/peds.2007-3709. Epub 2008 Jun 9. Pediatrics. 2008. PMID: 18541616 Free PMC article.
-
Plasma circulating nucleic acids levels increase according to the morbidity of Plasmodium vivax malaria.PLoS One. 2011;6(5):e19842. doi: 10.1371/journal.pone.0019842. Epub 2011 May 17. PLoS One. 2011. PMID: 21611202 Free PMC article.
-
Induced sputum proteome in healthy subjects and asthmatic patients.J Allergy Clin Immunol. 2011 Dec;128(6):1176-1184.e6. doi: 10.1016/j.jaci.2011.07.053. Epub 2011 Sep 8. J Allergy Clin Immunol. 2011. PMID: 21906793 Free PMC article.
References
-
- Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–S90. - PubMed
-
- Clark IA, Rockett KA. The cytokine theory of human cerebral malaria. Parasitol Today. 1994;10:410–412. - PubMed
-
- Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. - PubMed
-
- Adams S, Brown H, Turner G. Breaking down the blood-brain barrier: signaling a path to cerebral malaria? Trends Parasitol. 2002;18:360–366. - PubMed
-
- Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, Nagachinta B, White NJ, Berendt AR. An immunohistochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

