Pomc knockout mice have secondary hyperaldosteronism despite an absence of adrenocorticotropin
- PMID: 17991729
- PMCID: PMC2219304
- DOI: 10.1210/en.2006-1136
Pomc knockout mice have secondary hyperaldosteronism despite an absence of adrenocorticotropin
Abstract
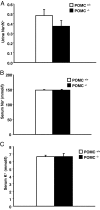
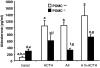
Aldosterone production is controlled by angiotensin II, potassium, and ACTH. Mice lacking Pomc and its pituitary product ACTH have been reported to have absent or low aldosterone levels, suggesting that ACTH is required for normal aldosterone production. However, this is at odds with the clinical finding that human aldosterone deficiency is not a component of secondary adrenal insufficiency. To resolve this, we measured plasma and urine electrolytes, together with plasma aldosterone and renin activity, in Pomc(-/-) mice. We found that these mice have secondary hyperaldosteronism (elevated aldosterone without suppression of renin activity), indicating that ACTH is not required for aldosterone production or release in vivo. Exogenous ACTH stimulates a further increase in aldosterone in Pomc(-/-) mice, whereas angiotensin II has no effect, and the combination of angiotensin II and ACTH is no more potent than ACTH alone. These data suggest that aldosterone production and release in vivo do not require the action of ACTH during development or postnatal life and that secondary hyperaldosteronism in Pomc(-/-) mice is a consequence of glucocorticoid deficiency.
Figures


Similar articles
-
Effect of the serotonin 5-HT4 receptor agonist cisapride on aldosterone secretion in corticotropic insufficiency and primary hyperaldosteronism.Neuroendocrinology. 1997 Sep;66(3):229-33. doi: 10.1159/000127242. Neuroendocrinology. 1997. PMID: 9380281 Clinical Trial.
-
Increased adrenal sensitivity to angiotensin II in idiopathic hyperaldosteronism.J Clin Endocrinol Metab. 1978 Nov;47(5):938-43. doi: 10.1210/jcem-47-5-938. J Clin Endocrinol Metab. 1978. PMID: 400738
-
In familial hyperaldosteronism type I, hybrid gene-induced aldosterone production dominates that induced by wild-type genes.J Clin Endocrinol Metab. 1997 Nov;82(11):3670-6. doi: 10.1210/jcem.82.11.4365. J Clin Endocrinol Metab. 1997. PMID: 9360524
-
Acute and chronic regulation of aldosterone production.Mol Cell Endocrinol. 2012 Mar 24;350(2):151-62. doi: 10.1016/j.mce.2011.07.034. Epub 2011 Aug 4. Mol Cell Endocrinol. 2012. PMID: 21839803 Free PMC article. Review.
-
Control of aldosterone secretion in domestic mammals during the perinatal period.Reprod Nutr Dev (1980). 1985;25(5):993-1005. doi: 10.1051/rnd:19850713. Reprod Nutr Dev (1980). 1985. PMID: 3001881 Review.
Cited by
-
Homozygous nonsense and frameshift mutations of the ACTH receptor in children with familial glucocorticoid deficiency (FGD) are not associated with long-term mineralocorticoid deficiency.Clin Endocrinol (Oxf). 2009 Aug;71(2):171-5. doi: 10.1111/j.1365-2265.2008.03511.x. Clin Endocrinol (Oxf). 2009. PMID: 19170705 Free PMC article.
-
Mineralocorticoid receptor activates postnatal adiposity in zebrafish lacking proopiomelanocortin.J Cell Physiol. 2024 Dec;239(12):e31428. doi: 10.1002/jcp.31428. Epub 2024 Sep 5. J Cell Physiol. 2024. PMID: 39238189 Free PMC article.
References
-
- Hajnoczky G, Csordas G, Bago A, Chiu AT, Spat A 1992 Angiotensin II exerts its effect on aldosterone production and potassium permeability through receptor subtype AT1 in rat adrenal glomerulosa cells. Biochem Pharmacol 43:1009–1012 - PubMed
-
- Ardaillou R 1999 Angiotensin II receptors. J Am Soc Nephrol 10(Suppl 11):S30–S39 - PubMed
-
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T 2000 International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472 - PubMed
-
- Yoshida A, Nishikawa T, Tamura Y, Yoshida S 1991 ACTH-induced inhibition of the action of angiotensin II in bovine zona glomerulosa cells. A modulatory effect of cyclic AMP on the angiotensin II receptor. J Biol Chem 266:4288–4294 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

