Dose- and rate-dependent effects of cocaine on striatal firing related to licking
- PMID: 17991811
- PMCID: PMC3160282
- DOI: 10.1124/jpet.107.129734
Dose- and rate-dependent effects of cocaine on striatal firing related to licking
Abstract
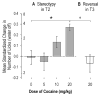
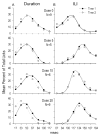
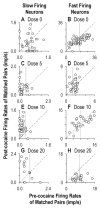
To examine the role of striatal mechanisms in cocaine-induced stereotyped licking, we investigated the acute effects of cocaine on striatal neurons in awake, freely moving rats before and after cocaine administration (0, 5, 10, or 20 mg/kg). Stereotyped licking was induced only by the high dose. Relative to control (saline), cocaine reduced lick duration and concurrently increased interlick interval, particularly at the high dose, but it did not affect licking rhythm. Firing rates of striatal neurons phasically related to licking movements were compared between matched licks before and after injection, minimizing any influence of sensorimotor variables on changes in firing. Both increases and decreases in average firing rate of striatal neurons were observed after cocaine injection, and these changes exhibited a dose-dependent pattern that strongly depended on predrug firing rate. At the middle and high doses relative to the saline group, the average firing rates of slow firing neurons were increased by cocaine, resulting from a general elevation of movement-related firing rates. In contrast, fast firing neurons showed decreased average firing rates only in the high-dose group, with reduced firing rates across the entire range for these neurons. Our findings suggest that at the high dose, increased phasic activity of slow firing striatal neurons and simultaneously reduced phasic activity of fast firing striatal neurons may contribute, respectively, to the continual initiation of stereotypic movements and the absence of longer movements.
Figures



Similar articles
-
Decreased firing of striatal neurons related to licking during acquisition and overtraining of a licking task.J Neurosci. 2009 Nov 4;29(44):13952-61. doi: 10.1523/JNEUROSCI.2824-09.2009. J Neurosci. 2009. PMID: 19890005 Free PMC article.
-
Amphetamine's dose-dependent effects on dorsolateral striatum sensorimotor neuron firing.Behav Brain Res. 2013 May 1;244:152-61. doi: 10.1016/j.bbr.2013.01.044. Epub 2013 Feb 8. Behav Brain Res. 2013. PMID: 23396149 Free PMC article.
-
Acute effects of cocaine on movement-related firing of dorsolateral striatal neurons depend on predrug firing rate and dose.J Pharmacol Exp Ther. 2010 Feb;332(2):667-83. doi: 10.1124/jpet.109.158253. Epub 2009 Nov 11. J Pharmacol Exp Ther. 2010. PMID: 19906778 Free PMC article.
-
Slow phasic and tonic activity of ventral pallidal neurons during cocaine self-administration.Synapse. 2012 Feb;66(2):106-27. doi: 10.1002/syn.20990. Epub 2011 Nov 3. Synapse. 2012. PMID: 21953543 Free PMC article.
-
Electrophysiological evidence of mediolateral functional dichotomy in the rat nucleus accumbens during cocaine self-administration II: phasic firing patterns.Eur J Neurosci. 2010 May;31(9):1671-82. doi: 10.1111/j.1460-9568.2010.07230.x. Eur J Neurosci. 2010. PMID: 20525080 Free PMC article.
Cited by
-
A procedure for implanting organized arrays of microwires for single-unit recordings in awake, behaving animals.J Vis Exp. 2014 Feb 14;(84):e51004. doi: 10.3791/51004. J Vis Exp. 2014. PMID: 24561332 Free PMC article.
-
The effects of a shared history of drug exposure on social choice.Behav Pharmacol. 2015 Oct;26(7 Spec No):631-5. doi: 10.1097/FBP.0000000000000139. Behav Pharmacol. 2015. PMID: 25932718 Free PMC article.
-
Decreased firing of striatal neurons related to licking during acquisition and overtraining of a licking task.J Neurosci. 2009 Nov 4;29(44):13952-61. doi: 10.1523/JNEUROSCI.2824-09.2009. J Neurosci. 2009. PMID: 19890005 Free PMC article.
-
Amphetamine's dose-dependent effects on dorsolateral striatum sensorimotor neuron firing.Behav Brain Res. 2013 May 1;244:152-61. doi: 10.1016/j.bbr.2013.01.044. Epub 2013 Feb 8. Behav Brain Res. 2013. PMID: 23396149 Free PMC article.
-
Dopamine transporter inhibition is required for cocaine-induced stereotypy.Neuroreport. 2008 Jul 16;19(11):1137-40. doi: 10.1097/WNR.0b013e3283063183. Neuroreport. 2008. PMID: 18596615 Free PMC article.
References
-
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. - PubMed
-
- Bhattacharyya AK, Pradhan SN. Interactions between motor activity and stereotypy in cocaine-treated rats. Psychopharmacology. 1979;63:311–312. - PubMed
-
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage Publications; Thousand Oaks, CA: 2002.
-
- Cho J, West MO. Distribution of single neurons related to body parts in the lateral striatum of the rat. Brain Res. 1997;756:241–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

