Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix
- PMID: 17991898
- PMCID: PMC2223433
- DOI: 10.1128/MCB.01766-07
Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix
Abstract
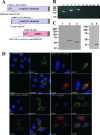
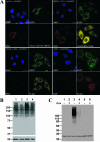
Recent discoveries of NAD-mediated regulatory processes in mitochondria have documented important roles of this compartmentalized nucleotide pool in addition to energy transduction. Moreover, mitochondria respond to excessive nuclear NAD consumption arising from DNA damage-induced poly-ADP-ribosylation because poly(ADP-ribose) (PAR) can trigger the release of apoptosis-inducing factor from the organelles. To functionally assess mitochondrial NAD metabolism, we overexpressed the catalytic domain of nuclear PAR polymerase 1 (PARP1) and targeted it to the matrix, which resulted in the constitutive presence of PAR within the organelles. As a result, stably transfected HEK293 cells exhibited a decrease in NAD content and typical features of respiratory deficiency. Remarkably, inhibiting PARP activity revealed PAR degradation within mitochondria. Two enzymes, PAR glycohydrolase (PARG) and ADP-ribosylhydrolase 3 (ARH3), are known to cleave PAR. Both full-length ARH3 and a PARG isoform, which arises from alternative splicing, localized to the mitochondrial matrix. This conclusion was based on the direct demonstration of their PAR-degrading activity within mitochondria of living cells. The visualization of catalytic activity establishes a new approach to identify submitochondrial localization of proteins involved in the metabolism of NAD derivatives. In addition, targeted PARP expression may serve as a compartment-specific "knock-down" of the NAD content which is readily detectable by PAR formation.
Figures




Similar articles
-
ADP-ribosylhydrolase 3 (ARH3), not poly(ADP-ribose) glycohydrolase (PARG) isoforms, is responsible for degradation of mitochondrial matrix-associated poly(ADP-ribose).J Biol Chem. 2012 May 11;287(20):16088-102. doi: 10.1074/jbc.M112.349183. Epub 2012 Mar 20. J Biol Chem. 2012. PMID: 22433848 Free PMC article.
-
PARP enzyme de novo synthesis of protein-free poly(ADP-ribose).Mol Cell. 2024 Dec 19;84(24):4758-4773.e6. doi: 10.1016/j.molcel.2024.10.024. Epub 2024 Nov 12. Mol Cell. 2024. PMID: 39536748
-
Functional Role of ADP-Ribosyl-Acceptor Hydrolase 3 in poly(ADP-Ribose) Polymerase-1 Response to Oxidative Stress.Curr Protein Pept Sci. 2016;17(7):633-640. doi: 10.2174/1389203717666160419144603. Curr Protein Pept Sci. 2016. PMID: 27090906 Free PMC article. Review.
-
New Insights into the Roles of NAD+-Poly(ADP-ribose) Metabolism and Poly(ADP-ribose) Glycohydrolase.Curr Protein Pept Sci. 2016;17(7):668-682. doi: 10.2174/1389203717666160419150014. Curr Protein Pept Sci. 2016. PMID: 27817743 Review.
-
Altered poly(ADP-ribose) metabolism impairs cellular responses to genotoxic stress in a hypomorphic mutant of poly(ADP-ribose) glycohydrolase.Exp Cell Res. 2007 Mar 10;313(5):984-96. doi: 10.1016/j.yexcr.2006.12.025. Epub 2007 Jan 10. Exp Cell Res. 2007. PMID: 17276427
Cited by
-
Protein and RNA ADP-ribosylation detection is influenced by sample preparation and reagents used.Life Sci Alliance. 2022 Nov 11;6(1):e202201455. doi: 10.26508/lsa.202201455. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36368907 Free PMC article.
-
Emerging roles of ADP-ribosyl-acceptor hydrolases (ARHs) in tumorigenesis and cell death pathways.Biochem Pharmacol. 2019 Sep;167:44-49. doi: 10.1016/j.bcp.2018.09.028. Epub 2018 Sep 27. Biochem Pharmacol. 2019. PMID: 30267646 Free PMC article. Review.
-
Post-translational modifications in mitochondria: protein signaling in the powerhouse.Cell Mol Life Sci. 2016 Nov;73(21):4063-73. doi: 10.1007/s00018-016-2280-4. Epub 2016 May 27. Cell Mol Life Sci. 2016. PMID: 27233499 Free PMC article. Review.
-
Pleiotropic cellular functions of PARP1 in longevity and aging: genome maintenance meets inflammation.Oxid Med Cell Longev. 2012;2012:321653. doi: 10.1155/2012/321653. Epub 2012 Sep 19. Oxid Med Cell Longev. 2012. PMID: 23050038 Free PMC article. Review.
-
Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes.Antioxid Redox Signal. 2019 Jan 10;30(2):251-294. doi: 10.1089/ars.2017.7269. Epub 2018 May 11. Antioxid Redox Signal. 2019. PMID: 29634344 Free PMC article. Review.
References
-
- Alano, C. C., W. Ying, and R. A. Swanson. 2004. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 27918895-18902. - PubMed
-
- Alvarez-Gonzalez, R., and M. K. Jacobson. 1987. Characterization of polymers of adenosine diphosphate ribose generated in vitro and in vivo. Biochemistry 263218-3224. - PubMed
-
- Ame, J. C., C. Spenlehauer, and G. de Murcia. 2004. The PARP superfamily. Bioessays 26882-893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
