Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks
- PMID: 17991920
- PMCID: PMC4920473
- DOI: 10.1200/JCO.2007.11.3324
Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks
Abstract
Purpose: The objective of this report is to provide a historical overview of and the issues and challenges inherent in the incorporation of patient-reported outcomes (PROs) into multinational cancer clinical trials in the cancer cooperative groups.
Methods: An online survey of 12 cancer cooperative groups from the United States, Canada, and Europe was conducted between June and August of 2006. Each of the cooperative groups designated one respondent, who was a member of one of the PRO committees within the cooperative group.
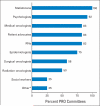
Results: There was a 100% response rate, and all of the cancer clinical trial cooperative groups reported conducting PRO research. PRO research has been conducted in the cancer cooperative groups for an average of 15 years (range, 6 to 30 years), and all groups had multidisciplinary committees focused on the design of PRO end points and the choice of appropriate PRO measures for cancer clinical trials. The cooperative groups reported that 5% to 50% of cancer treatment trials and an estimated 50% to 75% of cancer control trials contained PRO primary and secondary end points. There was considerable heterogeneity among the cooperative groups with respect to the formal and informal policies and procedures or cooperative group culture towards PROs, investigator training/mentorship, and resource availability for the measurement and conduct of PRO research within the individual cooperatives.
Conclusion: The challenges faced by the cooperative groups to the incorporation of PROs into cancer clinical trials are varied. Some common opportunities for improvement include the adoption of standardized training/mentorship mechanisms for investigators for the conduct of PRO assessments and data collection and the development of minimal criteria for PRO measure acceptability. A positive cultural shift has occurred in most of the cooperative groups related to the incorporation of PROs in clinical trials; however, financial and other resource barriers remain and need to be addressed.
Figures
Similar articles
-
Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks.J Clin Oncol. 2007 Nov 10;25(32):5070-7. doi: 10.1200/JCO.2007.12.7670. J Clin Oncol. 2007. PMID: 17991923 Review.
-
Use of patient-reported outcomes in phase III cancer treatment trials: lessons learned and future directions.J Clin Oncol. 2007 Nov 10;25(32):5063-9. doi: 10.1200/JCO.2007.11.0197. J Clin Oncol. 2007. PMID: 17991922 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Funding patient-reported outcomes in cancer clinical trials.J Clin Oncol. 2007 Nov 10;25(32):5100-5. doi: 10.1200/JCO.2007.11.5329. J Clin Oncol. 2007. PMID: 17991928 Review.
-
Patient-reported outcomes assessment in cancer trials: evaluating and enhancing the payoff to decision making.J Clin Oncol. 2007 Nov 10;25(32):5049-50. doi: 10.1200/JCO.2007.14.5888. J Clin Oncol. 2007. PMID: 17991919 No abstract available.
Cited by
-
Depression screening in patients with brain tumors: a review.CNS Oncol. 2015;4(2):71-8. doi: 10.2217/cns.14.60. CNS Oncol. 2015. PMID: 25768331 Free PMC article. Review.
-
In proportion: approaches for displaying patient-reported outcome research study results as percentages responding to treatment.Qual Life Res. 2019 Mar;28(3):609-620. doi: 10.1007/s11136-018-2065-3. Epub 2018 Nov 29. Qual Life Res. 2019. PMID: 30498892 Free PMC article.
-
Comparison of provider-assessed and patient-reported outcome measures of acute skin toxicity during a Phase III trial of mometasone cream versus placebo during breast radiotherapy: the North Central Cancer Treatment Group (N06C4).Int J Radiat Oncol Biol Phys. 2011 Oct 1;81(2):397-402. doi: 10.1016/j.ijrobp.2010.05.065. Epub 2010 Oct 1. Int J Radiat Oncol Biol Phys. 2011. PMID: 20888137 Free PMC article. Clinical Trial.
-
Children's Oncology Group's 2013 blueprint for research: nursing discipline.Pediatr Blood Cancer. 2013 Jun;60(6):1031-6. doi: 10.1002/pbc.24415. Epub 2012 Dec 19. Pediatr Blood Cancer. 2013. PMID: 23255369 Free PMC article. Review.
-
A depression network caused by brain tumours.Brain Struct Funct. 2022 Nov;227(8):2787-2795. doi: 10.1007/s00429-022-02573-z. Epub 2022 Oct 3. Brain Struct Funct. 2022. PMID: 36190539 Free PMC article.
References
-
- Johnson JR, Temple R. Food and Drug Administration requirements for approval of new anticancer drugs. Cancer Treat Rep. 1985;69:1155–1159. - PubMed
-
- Clinical Trials Cooperative Group Program-Cancer Therapy Evaluation Program: Guidelines. Division of Cancer Treatment, National Cancer Institute; Bethesda, MD: 1988.
-
- Trimble EL, Rowland J, Varricchio C, et al. Clinical trials referral resource: Health-related quality of life in cancer clinical trials. Oncology (Williston Park) 2001;15:456–458. 461–466. - PubMed
-
- Trimble EL, Rowland J, Varricchio C, et al. Clinical trials referral resource: Health-related quality of life in cancer clinical trials. Oncology (Williston Park) 2001;15:601–603. 606–608, 611. - PubMed
-
- Rowland JH, Varricchio CG, Trimble EL, et al. Clinical trials referral resource: Investigator-initiated health-related quality-of-life research. Oncology (Willis-ton Park) 2001;15:1284, 1286–1287, 1291–1292, 1294. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous