Identification of the prosurvival activity of nerve growth factor on cardiac myocytes
- PMID: 17992191
- PMCID: PMC2831217
- DOI: 10.1038/sj.cdd.4402263
Identification of the prosurvival activity of nerve growth factor on cardiac myocytes
Abstract
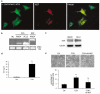
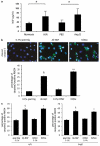
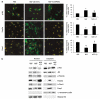
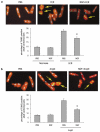
Neurotrophins (NTs) control neuron survival and regeneration. Recent research showed that NTs possess cardiovascular actions. In this study, we investigated the hypothesis that the NT nerve growth factor (NGF) prevents cardiomyocyte apoptosis. We demonstrated that cultured rat neonatal cardiomyocytes (RNCMs) produce NGF and express its trkA (tropomyosin-related receptor A (NGF high-affinity receptor)) receptor. RNCMs given a neutralizing antibody for NGF or the trkA inhibitor K252a underwent apoptosis, thus suggesting that NGF is an endogenous prosurvival factor for cardiomyocytes. Adenovirus (Ad)-mediated NGF overexpression protected RNCMs from apoptosis induced by either hypoxia/reoxygenation or angiotensin II (AngII). Similarly, recombinant NGF inhibited AngII-induced apoptosis in isolated rat adult cardiomyocytes. Finally, in a rat model of myocardial infarction, NGF gene transfer promoted cardiomyocyte survival. In RNCMs, recombinant NGF induced trkA phosphorylation, followed by Ser473 phosphorylation and nuclear translocation of phospho-protein kinase B (Akt). In response to Akt activation, Forkhead transcription factors Foxo-3a and Foxo-1 were phosphorylated and excluded from the nucleus. The prosurvival effect of adenoviral vector carrying the human NGF gene was inhibited in vitro by K252a, LY294002 (a pan-phosphatidyl inositol 3-kinase - PI3K - inhibitor), an Akt small interfering RNA, and adenoviruses carrying a dominant negative mutant form of Akt (Ad.DN.Akt) or an Akt-resistant Foxo-3a (Ad.AAA-Foxo-3a). These results newly demonstrate the cardiac prosurvival action of NGF and provide mechanistic information on the signaling pathway, which encompasses trkA, PI3K-Akt, and Foxo.
Figures




References
-
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. - PubMed
-
- Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. - PubMed
-
- Cantarella G, Lempeur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, et al. Nerve growth factor–endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. - PubMed
-
- Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

