von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis
- PMID: 17992257
- PMCID: PMC2066197
- DOI: 10.1172/JCI32614
von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis
Abstract
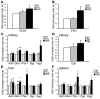
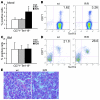
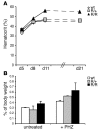
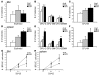
The R200W mutation in the von Hippel-Lindau (VHL) tumor suppressor protein (pVHL) is unique in that it is not associated with tumor development, but rather with Chuvash polycythemia, a heritable disease characterized by elevated hematocrit and increased serum levels of erythropoietin and VEGF. Previous studies have implicated hypoxia-inducible factor-1alpha (HIF-1alpha) signaling in this disorder, although the effects of this mutation on pVHL function are not fully understood. In order to explore the mechanisms underlying the development of this polycythemia, we generated mice homozygous for the R200W mutation (Vhl(R/R)). Vhl(R/R) mice developed polycythemia highly similar to the human disease. The activity of HIF proteins, specifically the HIF-2alpha isoform, was upregulated in ES cells and tissues from Vhl(R/R) mice. Furthermore, we observed a striking phenotype in Vhl(R/R) spleens, with greater numbers of erythroid progenitors and megakaryocytes and increased erythroid differentiation of Vhl(R/R) splenic cells in vitro. These findings suggest that enhanced expression of key HIF-2alpha genes promotes splenic erythropoiesis, resulting in the development of polycythemia in Vhl(R/R) mice. This mouse model is a faithful recapitulation of this VHL-associated syndrome and represents a useful tool for studying polycythemias and investigating potential therapeutics.
Figures
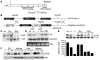
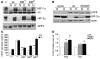


Similar articles
-
The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice.J Clin Invest. 2010 Mar;120(3):827-39. doi: 10.1172/JCI36362. Epub 2010 Feb 8. J Clin Invest. 2010. PMID: 20197624 Free PMC article.
-
Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia.Blood. 2009 Jun 18;113(25):6449-60. doi: 10.1182/blood-2008-07-167890. Epub 2009 Mar 20. Blood. 2009. PMID: 19304954
-
The phenotype of polycythemia due to Croatian homozygous VHL (571C>G:H191D) mutation is different from that of Chuvash polycythemia (VHL 598C>T:R200W).Haematologica. 2013 Apr;98(4):560-7. doi: 10.3324/haematol.2012.070508. Epub 2013 Feb 12. Haematologica. 2013. PMID: 23403324 Free PMC article.
-
Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis.Blood. 2009 Sep 3;114(10):2015-9. doi: 10.1182/blood-2009-05-189985. Epub 2009 Jun 3. Blood. 2009. PMID: 19494350 Review.
-
Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer.Eur Urol. 2016 Apr;69(4):646-657. doi: 10.1016/j.eururo.2015.08.007. Epub 2015 Aug 19. Eur Urol. 2016. PMID: 26298207 Free PMC article. Review.
Cited by
-
Altered cytokine profiles in patients with Chuvash polycythemia.Am J Hematol. 2009 Feb;84(2):74-8. doi: 10.1002/ajh.21327. Am J Hematol. 2009. PMID: 19062180 Free PMC article.
-
Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers.J Clin Invest. 2019 Feb 1;129(2):442-451. doi: 10.1172/JCI120855. Epub 2019 Jan 7. J Clin Invest. 2019. PMID: 30614813 Free PMC article. Review.
-
Hypoxia regulates BMP4 expression in the murine spleen during the recovery from acute anemia.PLoS One. 2010 Jun 24;5(6):e11303. doi: 10.1371/journal.pone.0011303. PLoS One. 2010. PMID: 20585586 Free PMC article.
-
Von Hippel-Lindau syndrome: molecular mechanisms of the disease.Clin Transl Oncol. 2010 Mar;12(3):160-5. doi: 10.1007/s12094-010-0485-9. Clin Transl Oncol. 2010. PMID: 20231120 Review.
-
The impact of O2 availability on human cancer.Nat Rev Cancer. 2008 Dec;8(12):967-75. doi: 10.1038/nrc2540. Epub 2008 Nov 6. Nat Rev Cancer. 2008. PMID: 18987634 Free PMC article. Review.
References
-
- Maxwell P.H., et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. - PubMed
-
- Ohh M., et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000;2:423–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

