Iron-binding properties of plant phenolics and cranberry's bio-effects
- PMID: 17992280
- PMCID: PMC2645657
- DOI: 10.1039/b705136k
Iron-binding properties of plant phenolics and cranberry's bio-effects
Abstract
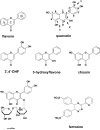
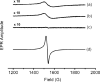
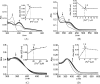
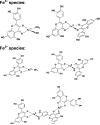
The health benefits of cranberries have long been recognized. However, the mechanisms behind its function are poorly understood. We have investigated the iron-binding properties of quercetin, the major phenolic phytochemical present in cranberries, and other selected phenolic compounds (chrysin, 3-hydroxyflavone, 3',4'-dihydroxy flavone, rutin, and flavone) in aqueous media using UV/vis, NMR and EPR spectroscopies and ESI-Mass spectrometry. Strong iron-binding properties have been confirmed for the compounds containing the "iron-binding motifs" identified in their structures. The apparent binding constants are estimated to be in the range of 10(6) M(-1) to 10(12) M(-2) in phosphate buffer at pH 7.2. Surprisingly, quercetin binds Fe(2+) even stronger than the well known Fe(2+)-chelator ferrozine at pH 7.2. This may be the first example of an oxygen-based ligand displaying stronger Fe(2+)-binding affinity than a strong nitrogen-based Fe(2+)-chelator. The strong Fe-binding properties of these phenolics argue that they may be effective in modulating cellular iron homeostasis under physiological conditions. Quercetin can completely suppress Fenton chemistry both at micromolar levels and in the presence of major cellular iron chelators like ATP or citrate. However, the radical scavenging activity of quercetin provides only partial protection against Fenton chemistry-mediated damage while Fe chelation by quercetin can completely inhibit Fenton chemistry, indicating that the chelation may be key to its antioxidant activity. These results demonstrate that quercetin and other phenolic compounds can effectively modulate iron biochemistry under physiologically relevant conditions, providing insight into the mechanism of action of bio-active phenolics.
Figures








Similar articles
-
Iron-binding and anti-Fenton properties of baicalein and baicalin.J Inorg Biochem. 2009 Mar;103(3):326-32. doi: 10.1016/j.jinorgbio.2008.11.003. Epub 2008 Nov 19. J Inorg Biochem. 2009. PMID: 19108897 Free PMC article.
-
pH-specific synthetic chemistry and solution studies in the binary system of iron(III) with the alpha-hydroxycarboxylate substrate quinic acid: potential relevance to iron chemistry in plant fluids.Inorg Chem. 2009 Mar 2;48(5):1844-56. doi: 10.1021/ic800356v. Inorg Chem. 2009. PMID: 19235948
-
On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex.Biometals. 2000 Mar;13(1):77-83. doi: 10.1023/a:1009229429250. Biometals. 2000. PMID: 10831228
-
Quercetin and iron metabolism: What we know and what we need to know.Food Chem Toxicol. 2018 Apr;114:190-203. doi: 10.1016/j.fct.2018.02.022. Epub 2018 Feb 10. Food Chem Toxicol. 2018. PMID: 29432835 Review.
-
Cranberries: ripe for more cancer research?J Sci Food Agric. 2011 Oct;91(13):2303-7. doi: 10.1002/jsfa.4621. Epub 2011 Aug 30. J Sci Food Agric. 2011. PMID: 21910124 Review.
Cited by
-
Bauhinia forficata prevents vacuous chewing movements induced by haloperidol in rats and has antioxidant potential in vitro.Neurochem Res. 2013 Apr;38(4):789-96. doi: 10.1007/s11064-013-0981-8. Epub 2013 Feb 2. Neurochem Res. 2013. PMID: 23377855
-
Selected Natural Products in Neuroprotective Strategies for Alzheimer's Disease-A Non-Systematic Review.Int J Mol Sci. 2022 Jan 21;23(3):1212. doi: 10.3390/ijms23031212. Int J Mol Sci. 2022. PMID: 35163136 Free PMC article. Review.
-
Iron-binding and anti-Fenton properties of baicalein and baicalin.J Inorg Biochem. 2009 Mar;103(3):326-32. doi: 10.1016/j.jinorgbio.2008.11.003. Epub 2008 Nov 19. J Inorg Biochem. 2009. PMID: 19108897 Free PMC article.
-
Enhancing the potency of lithospermate B for inhibiting Na+/K+-ATPase activity by forming transition metal ion complexes.Acta Pharmacol Sin. 2013 Jul;34(7):893-900. doi: 10.1038/aps.2013.32. Epub 2013 May 20. Acta Pharmacol Sin. 2013. PMID: 23685954 Free PMC article.
-
Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential.Antioxidants (Basel). 2019 Sep 1;8(9):355. doi: 10.3390/antiox8090355. Antioxidants (Basel). 2019. PMID: 31480581 Free PMC article.
References
-
- Blatherwick NR. Arch. Intern. Med. 1914;14:409–450.
-
- Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskil I, Lipsitz LA. J. Am. Med. Assoc. 1994;271:751–754. - PubMed
-
- Neto CC. J. Nutr. 2007;137:186S–193S. - PubMed
-
- Neto CC, Krueger CG, Lamoureaux TL, Kondo M, Vaisberg AJ, Hurta RAR, Curtis S, Matchett MD, Yeung H, Sweeney MI, Reed JD. J. Sci. Food Agric. 2006;86:18–25.
-
- Weiss EI, Houri-Haddad Y, Greenbaum E, Hochman N, Ofek I, Zakay-Rones Z. Antiviral Res. 2005;66:9–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

