How do HYNIC-conjugated peptides bind technetium? Insights from LC-MS and stability studies
- PMID: 17992285
- PMCID: PMC2258084
- DOI: 10.1039/b705111e
How do HYNIC-conjugated peptides bind technetium? Insights from LC-MS and stability studies
Abstract
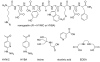
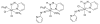
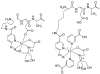
Hydrazinonicotinamide (HYNIC) is an established bifunctional complexing agent for technetium-99m ((99m)Tc) but the structure of the technetium coordination sphere remains uncertain. To gain further insight into this, we have prepared conjugates of HYNIC and hydrazinobenzoic acid (HYBA) with a model peptide, and radiolabelled them with (99m)Tc using three well-established co-ligand systems: EDDA, tricine and tricine-nicotinic acid. The labelled peptides were studied by LC-MS and by subjecting them to serum stability and protein binding assays. For each co-ligand system, HYNIC conjugates formed fewer and more stable labelled species than the corresponding HYBA conjugates. LC-MS analysis showed that all conjugates contained one hydrazine moiety bound to Tc, that binding of Tc to HYNIC-peptide and co-ligand occurs with displacement of 5H(+) indicating a Tc formal oxidation state of +5, and that the Tc has no oxo- or halide ligands. LC-MS also shows that complexes formed with the HYNIC conjugate contain fewer coordinating co-ligand molecules than the HYBA conjugate indicating that HYNIC is able to more effectively satisfy the coordination requirement of technetium, perhaps by binding in chelating mode.
Figures
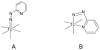




Similar articles
-
Technetium-binding in labelled HYNIC-peptide conjugates: role of coordinating amino acids.J Inorg Biochem. 2009 Jul;103(7):971-7. doi: 10.1016/j.jinorgbio.2009.04.007. Epub 2009 May 3. J Inorg Biochem. 2009. PMID: 19447500
-
Synthesis and evaluation of analogues of HYNIC as bifunctional chelators for technetium.Dalton Trans. 2011 Jun 21;40(23):6260-7. doi: 10.1039/c0dt01608j. Epub 2011 Feb 25. Dalton Trans. 2011. PMID: 21350776
-
Technetium-99m somatostatin analogues: effect of labelling methods and peptide sequence.Eur J Nucl Med. 1999 Aug;26(8):869-76. doi: 10.1007/s002590050461. Eur J Nucl Med. 1999. PMID: 10436200
-
99mTc-Nicotinic acid/tricine/hydrazinonicotinamide-nonsulfated cholecystokinin-8.2007 Aug 31 [updated 2007 Sep 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Aug 31 [updated 2007 Sep 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641933 Free Books & Documents. Review.
-
Study of the 99m Tc-labeling conditions of 6-hydrazinonicotinamide-conjugated peptides from a new perspective: Introduction to the term radio-stoichiometry.J Labelled Comp Radiopharm. 2020 Dec;63(14):582-596. doi: 10.1002/jlcr.3883. Epub 2020 Oct 30. J Labelled Comp Radiopharm. 2020. PMID: 32997359 Review.
Cited by
-
Opportunities and challenges for metal chemistry in molecular imaging: from gamma camera imaging to PET and multimodality imaging.Adv Inorg Chem. 2016;68:1-41. doi: 10.1016/bs.adioch.2015.09.001. Epub 2015 Nov 16. Adv Inorg Chem. 2016. PMID: 30381783 Free PMC article.
-
99mTc-HYNIC-spermine for imaging polyamine transport system-positive tumours: preclinical evaluation.Eur J Nucl Med Mol Imaging. 2011 Oct;38(10):1832-41. doi: 10.1007/s00259-011-1857-2. Epub 2011 Jun 10. Eur J Nucl Med Mol Imaging. 2011. PMID: 21660624
-
Synthesis and preliminary evaluation of a new (99m)tc labeled substance p analogue as a potential tumor imaging agent.Iran J Pharm Res. 2015 Winter;14(1):97-110. Iran J Pharm Res. 2015. PMID: 25561916 Free PMC article.
-
Synthesis and Evaluation of 99mTc-Labeled PSMA-Targeted Tracers Based on the Lys-Urea-Aad Pharmacophore for Detecting Prostate Cancer with Single Photon Emission Computed Tomography.Molecules. 2023 Jun 29;28(13):5120. doi: 10.3390/molecules28135120. Molecules. 2023. PMID: 37446782 Free PMC article.
-
Cholecystokinin-2 Receptor Targeting with Novel C-terminally Stabilized HYNIC-Minigastrin Analogs Radiolabeled with Technetium-99m.Pharmaceuticals (Basel). 2019 Jan 15;12(1):13. doi: 10.3390/ph12010013. Pharmaceuticals (Basel). 2019. PMID: 30650563 Free PMC article.
References
-
- de Jong M, Kwekkeboom D, Valkema R, Krenning EP. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:463. - PubMed
-
- Reubi JC. Endocr. Pathol. 1997;8:11. - PubMed
-
- Behe M, Behr TM. Biopolymers. 2002;66:399. - PubMed
-
- Behe M, Becker W, Gotthardt M, Angerstein C, Behr TM. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1140. - PubMed
-
- Banerjee S, Pillai MR, Ramamoorthy N. Semin. Nucl. Med. 2001;31:260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

