How do HYNIC-conjugated peptides bind technetium? Insights from LC-MS and stability studies
- PMID: 17992285
- PMCID: PMC2258084
- DOI: 10.1039/b705111e
How do HYNIC-conjugated peptides bind technetium? Insights from LC-MS and stability studies
Abstract
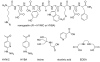
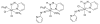
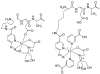
Hydrazinonicotinamide (HYNIC) is an established bifunctional complexing agent for technetium-99m ((99m)Tc) but the structure of the technetium coordination sphere remains uncertain. To gain further insight into this, we have prepared conjugates of HYNIC and hydrazinobenzoic acid (HYBA) with a model peptide, and radiolabelled them with (99m)Tc using three well-established co-ligand systems: EDDA, tricine and tricine-nicotinic acid. The labelled peptides were studied by LC-MS and by subjecting them to serum stability and protein binding assays. For each co-ligand system, HYNIC conjugates formed fewer and more stable labelled species than the corresponding HYBA conjugates. LC-MS analysis showed that all conjugates contained one hydrazine moiety bound to Tc, that binding of Tc to HYNIC-peptide and co-ligand occurs with displacement of 5H(+) indicating a Tc formal oxidation state of +5, and that the Tc has no oxo- or halide ligands. LC-MS also shows that complexes formed with the HYNIC conjugate contain fewer coordinating co-ligand molecules than the HYBA conjugate indicating that HYNIC is able to more effectively satisfy the coordination requirement of technetium, perhaps by binding in chelating mode.
Figures
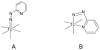




References
-
- de Jong M, Kwekkeboom D, Valkema R, Krenning EP. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:463. - PubMed
-
- Reubi JC. Endocr. Pathol. 1997;8:11. - PubMed
-
- Behe M, Behr TM. Biopolymers. 2002;66:399. - PubMed
-
- Behe M, Becker W, Gotthardt M, Angerstein C, Behr TM. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1140. - PubMed
-
- Banerjee S, Pillai MR, Ramamoorthy N. Semin. Nucl. Med. 2001;31:260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

