Spinal stenosis: assessment of motor function, VEGF expression and angiogenesis in an experimental model in the rat
- PMID: 17992557
- PMCID: PMC2223356
- DOI: 10.1007/s00586-007-0394-y
Spinal stenosis: assessment of motor function, VEGF expression and angiogenesis in an experimental model in the rat
Abstract
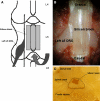
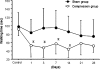
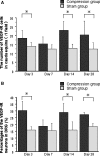
Reduction of blood flow in compressed nerve roots is considered as one important mechanism of induction of neurogenic intermittent claudication in lumbar spinal canal stenosis. Vascular endothelial growth factor (VEGF) is a potent stimulator of angiogenesis, and is increased in expression in hypoxic conditions. The objective of this study was to examine if cauda equina compression affects motor function and induces expression of VEGF and angiogenesis. The cauda equina was compressed by placing a piece of silicone rubber into the L5 epidural space. Walking duration was examined by rota-rod testing. The compressed parts of the cauda equina and L5 dorsal root ganglion (DRG) were removed at 3, 7, 14, or 28 days after surgery, and processed for immunohistochemistry for VEGF and Factor VIII (marker for vascular endothelial cells). Numbers of VEGF-immunoreactive (IR) cells and vascular density were examined. Walking duration was decreased after induction of cauda equina compression. The number of VEGF-IR cells in the cauda equina and DRG was significantly increased at 3, 14, and 28 days after cauda equina compression, compared with sham-operated rats (P < 0.05). Vascular density in the cauda equina was not increased at any of the time points examined. Cauda equina compression decreased walking duration, and induced VEGF expression in nerve roots and DRG.
Figures

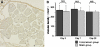
Similar articles
-
Compensatory neovascularization after cauda equina compression in rats.Spine (Phila Pa 1976). 2008 Jan 15;33(2):140-5. doi: 10.1097/BRS.0b013e31816044d2. Spine (Phila Pa 1976). 2008. PMID: 18197097
-
Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis.Spine (Phila Pa 1976). 2007 Jan 15;32(2):159-67. doi: 10.1097/01.brs.0000251437.10545.e9. Spine (Phila Pa 1976). 2007. PMID: 17224809
-
Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain.Spine (Phila Pa 1976). 2004 May 15;29(10):1105-11. doi: 10.1097/00007632-200405150-00011. Spine (Phila Pa 1976). 2004. PMID: 15131438
-
Spinal nerve root compression. Nutrition and function of the porcine cauda equina compressed in vivo.Acta Orthop Scand Suppl. 1991;242:1-27. Acta Orthop Scand Suppl. 1991. PMID: 1645923 Review.
-
[Blood circulation of cauda equina and nerve root].Clin Calcium. 2005 Mar;15(3):63-72. Clin Calcium. 2005. PMID: 15741681 Review. Japanese.
Cited by
-
Simvastatin ameliorates cauda equina compression injury in a rat model of lumbar spinal stenosis.J Neuroimmune Pharmacol. 2013 Mar;8(1):274-86. doi: 10.1007/s11481-012-9419-3. Epub 2012 Nov 28. J Neuroimmune Pharmacol. 2013. PMID: 23188522 Free PMC article.
-
Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: A study using a rat cauda equina compression model.Sci Rep. 2019 Nov 12;9(1):16578. doi: 10.1038/s41598-019-52999-5. Sci Rep. 2019. PMID: 31719574 Free PMC article.
-
Animal Models of Intervertebral Disc Diseases: Advantages, Limitations, and Future Directions.Neurol Int. 2024 Dec 9;16(6):1788-1818. doi: 10.3390/neurolint16060129. Neurol Int. 2024. PMID: 39728755 Free PMC article. Review.
-
S-Nitrosoglutathione administration ameliorates cauda equina compression injury in rats.Neurosci Med. 2012 Sep 25;3(3):294-305. doi: 10.4236/nm.2012.33034. Neurosci Med. 2012. PMID: 23997981 Free PMC article.
-
Transforming Growth Factor-β Induces Interleukin-6 Secretion from Human Ligamentum Flavum-Derived Cells through Partial Activation of p38 and p44/42 Mitogen-Activated Protein Kinases.Asian Spine J. 2023 Dec;17(6):997-1003. doi: 10.31616/asj.2023.0025. Epub 2023 Nov 10. Asian Spine J. 2023. PMID: 37946333 Free PMC article.
References
-
- Baker AR, Collins TA, Poter RW, et al. Laser doppler study of procaine cauda equina blood flow. The effect of electrical stimulation of the rootlets during single and double site, low pressure compression of the cauda equina. Spine. 1995;20:660–664. doi: 10.1097/00007632-199503150-00005. - DOI - PubMed
-
- Hayashi T, Abe K, Suzuki H, et al. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

