A new autocatalytic activation mechanism for cysteine proteases revealed by Prevotella intermedia interpain A
- PMID: 17993455
- PMCID: PMC2772895
- DOI: 10.1074/jbc.M708481200
A new autocatalytic activation mechanism for cysteine proteases revealed by Prevotella intermedia interpain A
Abstract
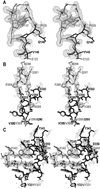
Prevotella intermedia is a major periodontopathogen contributing to human gingivitis and periodontitis. Such pathogens release proteases as virulence factors that cause deterrence of host defenses and tissue destruction. A new cysteine protease from the cysteine-histidine-dyad class, interpain A, was studied in its zymogenic and self-processed mature forms. The latter consists of a bivalved moiety made up by two subdomains. In the structure of a catalytic cysteine-to-alanine zymogen variant, the right subdomain interacts with an unusual prodomain, thus contributing to latency. Unlike the catalytic cysteine residue, already in its competent conformation in the zymogen, the catalytic histidine is swung out from its active conformation and trapped in a cage shaped by a backing helix, a zymogenic hairpin, and a latency flap in the zymogen. Dramatic rearrangement of up to 20A of these elements triggered by a tryptophan switch occurs during activation and accounts for a new activation mechanism for proteolytic enzymes. These findings can be extrapolated to related potentially pathogenic cysteine proteases such as Streprococcus pyogenes SpeB and Porphyromonas gingivalis periodontain.
Figures


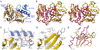

Similar articles
-
Autocatalytic processing of the streptococcal cysteine protease zymogen: processing mechanism and characterization of the autoproteolytic cleavage sites.Eur J Biochem. 1999 Jul;263(1):145-51. doi: 10.1046/j.1432-1327.1999.00473.x. Eur J Biochem. 1999. PMID: 10429198
-
Structural insights unravel the zymogenic mechanism of the virulence factor gingipain K from Porphyromonas gingivalis, a causative agent of gum disease from the human oral microbiome.J Biol Chem. 2017 Apr 7;292(14):5724-5735. doi: 10.1074/jbc.M117.776724. Epub 2017 Feb 14. J Biol Chem. 2017. PMID: 28196869 Free PMC article.
-
C-Terminal extension of a plant cysteine protease modulates proteolytic activity through a partial inhibitory mechanism.FEBS J. 2011 Sep;278(17):3012-24. doi: 10.1111/j.1742-4658.2011.08221.x. Epub 2011 Jul 18. FEBS J. 2011. PMID: 21707922
-
Structural aspects of activation pathways of aspartic protease zymogens and viral 3C protease precursors.Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):10968-75. doi: 10.1073/pnas.96.20.10968. Proc Natl Acad Sci U S A. 1999. PMID: 10500110 Free PMC article. Review.
-
Catalytic mechanism of the 20S proteasome of Thermoplasma acidophilum revealed by X-ray crystallography.Cold Spring Harb Symp Quant Biol. 1995;60:525-32. doi: 10.1101/sqb.1995.060.01.056. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824425 Review. No abstract available.
Cited by
-
Solution structure and backbone dynamics of streptopain: insight into diverse substrate specificity.J Biol Chem. 2009 Apr 17;284(16):10957-67. doi: 10.1074/jbc.M807624200. Epub 2009 Feb 23. J Biol Chem. 2009. PMID: 19237546 Free PMC article.
-
Ultratight crystal packing of a 10 kDa protein.Acta Crystallogr D Biol Crystallogr. 2013 Mar;69(Pt 3):464-70. doi: 10.1107/S0907444912050135. Epub 2013 Feb 16. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 23519421 Free PMC article.
-
Saccharomyces cerevisiae Env7 is a novel serine/threonine kinase 16-related protein kinase and negatively regulates organelle fusion at the lysosomal vacuole.Mol Cell Biol. 2013 Feb;33(3):526-42. doi: 10.1128/MCB.01303-12. Epub 2012 Nov 19. Mol Cell Biol. 2013. PMID: 23166297 Free PMC article.
-
The dissemination of C10 cysteine protease genes in Bacteroides fragilis by mobile genetic elements.BMC Microbiol. 2010 Apr 23;10:122. doi: 10.1186/1471-2180-10-122. BMC Microbiol. 2010. PMID: 20416045 Free PMC article.
-
Designing Novel Multi-Epitope Vaccine Construct against Prevotella intermedia-Interpain A: An Immunoinformatics Approach.Medicina (Kaunas). 2023 Feb 6;59(2):302. doi: 10.3390/medicina59020302. Medicina (Kaunas). 2023. PMID: 36837503 Free PMC article.
References
-
- AAPHD-SPP. J. Public Health Dent. 1983;43:106–117. - PubMed
-
- Pihlstrom BL, Michalowicz BS, Johnson NW. Lancet. 2005;366:1809–1820. - PubMed
-
- Jordan RC. Periodontol. 2000. 2004;34:217–229. - PubMed
-
- Haffajee AD, Socransky SS, Gunsolley JC. Ann. Periodontol. 2003;8:115–181. - PubMed
-
- Tanner AC, Izard J. Periodontol. 2000. 2006;42:88–113. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

