PAR1 specifies ciliated cells in vertebrate ectoderm downstream of aPKC
- PMID: 17993468
- PMCID: PMC2170474
- DOI: 10.1242/dev.009282
PAR1 specifies ciliated cells in vertebrate ectoderm downstream of aPKC
Abstract
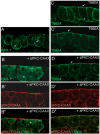
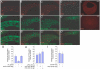
Partitioning-defective 1 (PAR1) and atypical protein kinase C (aPKC) are conserved serine/threonine protein kinases implicated in the establishment of cell polarity in many species from yeast to humans. Here we investigate the roles of these protein kinases in cell fate determination in Xenopus epidermis. Early asymmetric cell divisions at blastula and gastrula stages give rise to the superficial (apical) and the deep (basal) cell layers of epidermal ectoderm. These two layers consist of cells with different intrinsic developmental potential, including superficial epidermal cells and deep ciliated cells. Our gain- and loss-of-function studies demonstrate that aPKC inhibits ciliated cell differentiation in Xenopus ectoderm and promotes superficial cell fates. We find that the crucial molecular substrate for aPKC is PAR1, which is localized in a complementary domain in superficial ectoderm cells. We show that PAR1 acts downstream of aPKC and is sufficient to stimulate ciliated cell differentiation and inhibit superficial epidermal cell fates. Our results suggest that aPKC and PAR1 function sequentially in a conserved molecular pathway that links apical-basal cell polarity to Notch signaling and cell fate determination. The observed patterning mechanism may operate in a wide range of epithelial tissues in many species.
Figures






References
-
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. - PubMed
-
- Bayraktar J, Zygmunt D, Carthew RW. Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J. Cell Sci. 2006;119:711–721. - PubMed
-
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. - PubMed
-
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 2004;14:R674–R685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

