Surface rheology and adsorption kinetics reveal the relative amphiphilicity, interfacial activity, and stability of human exchangeable apolipoproteins
- PMID: 17993480
- PMCID: PMC2242762
- DOI: 10.1529/biophysj.107.115220
Surface rheology and adsorption kinetics reveal the relative amphiphilicity, interfacial activity, and stability of human exchangeable apolipoproteins
Abstract
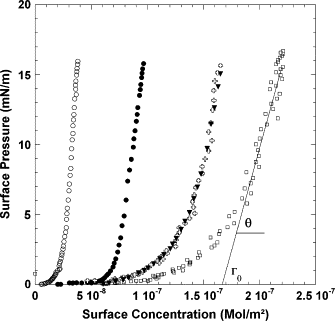
Exchangeable apolipoproteins are located in the surface of lipoprotein particles and regulate lipid metabolism through direct protein-protein and protein-lipid interactions. These proteins are characterized by the presence of tandem repeats of amphiphatic alpha-helix segments and a high surface activity in monolayers and lipoprotein surfaces. A noteworthy aspect in the description of the function of exchangeable apolipoproteins is the requirement of a quantitative account of the relation between their physicochemical and structural characteristics and changes in the mesoscopic system parameters such as the maximum surface pressure and relative stability at interfaces. To comply with this demand, we set out to establish the relations among alpha-helix amphiphilicity, surface concentration, and surface rheology of apolipoproteins ApoA-I, ApoA-II, ApoC-I, ApoC-II, and ApoC-III adsorbed at the air-water interface. Our studies render further insights into the interfacial properties of exchangeable apolipoproteins, including the kinetics of their adsorption and the physical properties of the interfacial layer.
Figures





Similar articles
-
Interfacial properties of an amphipathic alpha-helix consensus peptide of exchangeable apolipoproteins at air/water and oil/water interfaces.J Biol Chem. 2003 Sep 26;278(39):37480-91. doi: 10.1074/jbc.M303133200. Epub 2003 Jul 3. J Biol Chem. 2003. PMID: 12842901
-
The surface properties of apolipoproteins A-I and A-II at the lipid/water interface.Biochim Biophys Acta. 1989 Aug 22;1004(3):300-8. doi: 10.1016/0005-2760(89)90077-5. Biochim Biophys Acta. 1989. PMID: 2503030
-
Characterization of the tetratricopeptide-containing domain of BUB1, BUBR1, and PP5 proves that domain amphiphilicity over amino acid sequence specificity governs protein adsorption and interfacial activity.J Phys Chem B. 2008 Jul 10;112(27):7984-91. doi: 10.1021/jp711222s. Epub 2008 Jun 12. J Phys Chem B. 2008. PMID: 18547097
-
Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins.Prog Lipid Res. 2004 Jul;43(4):350-80. doi: 10.1016/j.plipres.2004.05.002. Prog Lipid Res. 2004. PMID: 15234552 Review.
-
Amyloid-Forming Properties of Human Apolipoproteins: Sequence Analyses and Structural Insights.Adv Exp Med Biol. 2015;855:175-211. doi: 10.1007/978-3-319-17344-3_8. Adv Exp Med Biol. 2015. PMID: 26149931 Free PMC article. Review.
Cited by
-
Do clustering monoclonal antibody solutions really have a concentration dependence of viscosity?Biophys J. 2013 Feb 19;104(4):913-23. doi: 10.1016/j.bpj.2013.01.007. Biophys J. 2013. PMID: 23442970 Free PMC article.
-
Apolipoprotein C-I binds more strongly to phospholipid/triolein/water than triolein/water interfaces: a possible model for inhibiting cholesterol ester transfer protein activity and triacylglycerol-rich lipoprotein uptake.Biochemistry. 2012 Feb 14;51(6):1238-48. doi: 10.1021/bi2015212. Epub 2012 Feb 2. Biochemistry. 2012. PMID: 22264166 Free PMC article.
-
A Pressure-dependent Model for the Regulation of Lipoprotein Lipase by Apolipoprotein C-II.J Biol Chem. 2015 Jul 17;290(29):18029-18044. doi: 10.1074/jbc.M114.629865. Epub 2015 May 29. J Biol Chem. 2015. PMID: 26026161 Free PMC article.
References
-
- Fielding C., Fielding P. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36:211–228. - PubMed
-
- Liu T., Krieger M., Kan H.-Y., Zannis V.I. The effects of mutations in helices 4 and 6 of ApoA-I on scavenger receptor class b type i (sr-bi)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and sr-bi is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. - PubMed
-
- Arakawa R., Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 2002;277:22426–22429. - PubMed
-
- Major A.S., Dove D.E., Ishiguro H., Su Y.R., Brown A.M., Liu L., Carter K.J., Linton M.F., Fazio S. Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI(−/−) mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:1790–1795. - PubMed
-
- Nishida H., Nakanishi T., Yen E., Arai H., Yen F., Nishida T. Nature of the enhancement of lecithin-cholesterol acyltransferase reaction by various apolipoproteins. J. Biol. Chem. 1986;261:12028–12035. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

