Defects in vesicle core induced by escherichia coli dihydroorotate dehydrogenase
- PMID: 17993483
- PMCID: PMC2242746
- DOI: 10.1529/biophysj.107.120055
Defects in vesicle core induced by escherichia coli dihydroorotate dehydrogenase
Abstract
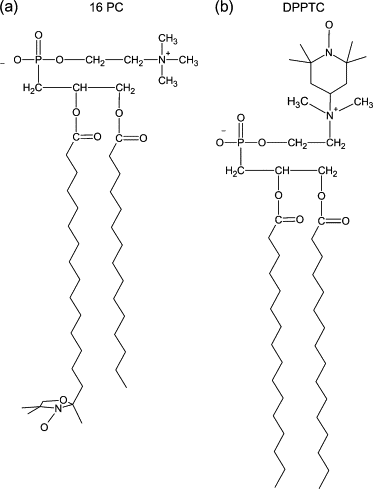
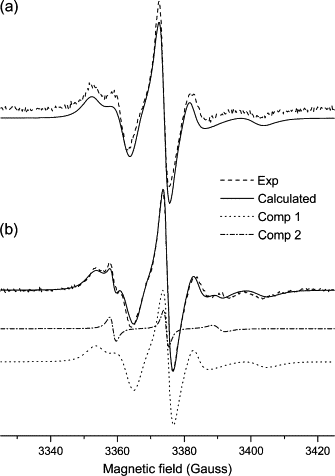
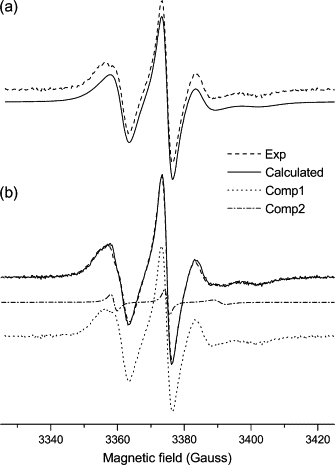
Dihydroorotate dehydrogenase (DHODH) catalyzes the oxidation of dihydroorotate to orotate during the fourth step of the de novo pyrimidine synthesis pathway. In rapidly proliferating mammalian cells, pyrimidine salvage pathway is insufficient to overcome deficiencies in that pathway for nucleotide synthesis. Moreover, as certain parasites lack salvage enzymes, relying solely on the de novo pathway, DHODH inhibition has turned out as an efficient way to block pyrimidine biosynthesis. Escherichia coli DHODH (EcDHODH) is a class 2 DHODH, found associated to cytosolic membranes through an N-terminal extension. We used electronic spin resonance (ESR) to study the interaction of EcDHODH with vesicles of 1,2-dioleoyl-sn-glycero-phosphatidylcholine/detergent. Changes in vesicle dynamic structure induced by the enzyme were monitored via spin labels located at different positions of phospholipid derivatives. Two-component ESR spectra are obtained for labels 5- and 10-phosphatidylcholine in presence of EcDHODH, whereas other probes show a single-component spectrum. The appearance of an additional spectral component with features related to fast-motion regime of the probe is attributed to the formation of a defect-like structure in the membrane hydrophobic region. This is probably the mechanism used by the protein to capture quinones used as electron acceptors during catalysis. The use of specific spectral simulation routines allows us to characterize the ESR spectra in terms of changes in polarity and mobility around the spin-labeled phospholipids. We believe this is the first report of direct evidences concerning the binding of class 2 DHODH to membrane systems.
Figures



Similar articles
-
Site directed spin labeling studies of Escherichia coli dihydroorotate dehydrogenase N-terminal extension.Biochem Biophys Res Commun. 2011 Oct 28;414(3):487-92. doi: 10.1016/j.bbrc.2011.09.092. Epub 2011 Oct 2. Biochem Biophys Res Commun. 2011. PMID: 21986535
-
Inhibitor binding in a class 2 dihydroorotate dehydrogenase causes variations in the membrane-associated N-terminal domain.Protein Sci. 2004 Apr;13(4):1031-42. doi: 10.1110/ps.03533004. Protein Sci. 2004. PMID: 15044733 Free PMC article.
-
Malarial dihydroorotate dehydrogenase. Substrate and inhibitor specificity.J Biol Chem. 2002 Nov 1;277(44):41827-34. doi: 10.1074/jbc.M206854200. Epub 2002 Aug 19. J Biol Chem. 2002. PMID: 12189151
-
Recent developments in the medicinal chemistry and therapeutic potential of dihydroorotate dehydrogenase (DHODH) inhibitors.Mini Rev Med Chem. 2011 Oct;11(12):1039-55. doi: 10.2174/138955711797247707. Mini Rev Med Chem. 2011. PMID: 21861807 Review.
-
Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer.Pharmacol Ther. 2019 Mar;195:111-131. doi: 10.1016/j.pharmthera.2018.10.012. Epub 2018 Oct 19. Pharmacol Ther. 2019. PMID: 30347213 Review.
Cited by
-
Conformational changes of the HsDHODH N-terminal Microdomain via DEER Spectroscopy.J Phys Chem B. 2015 Jul 16;119(28):8693-7. doi: 10.1021/acs.jpcb.5b01706. Epub 2015 Jul 2. J Phys Chem B. 2015. PMID: 26086954 Free PMC article.
-
Effects of GPI-anchored TNAP on the dynamic structure of model membranes.Phys Chem Chem Phys. 2015 Oct 21;17(39):26295-301. doi: 10.1039/c5cp02377g. Phys Chem Chem Phys. 2015. PMID: 26389140 Free PMC article.
-
Effects of Nicotine on the Thermodynamics and Phase Coexistence of Pulmonary Surfactant Model Membranes.Membranes (Basel). 2024 Dec 11;14(12):267. doi: 10.3390/membranes14120267. Membranes (Basel). 2024. PMID: 39728717 Free PMC article.
-
De Novo Pyrimidine Biosynthesis Connects Cell Integrity to Amphotericin B Susceptibility in Cryptococcus neoformans.mSphere. 2016 Nov 16;1(6):e00191-16. doi: 10.1128/mSphere.00191-16. eCollection 2016 Nov-Dec. mSphere. 2016. PMID: 27904878 Free PMC article.
-
Probing the interaction of brain fatty acid binding protein (B-FABP) with model membranes.PLoS One. 2013;8(3):e60198. doi: 10.1371/journal.pone.0060198. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555925 Free PMC article.
References
-
- Jones M.E. Pyrimidine nucleotide biosynthesis in animals: Genes, enzymes, and regulation of UMP biosynthesis. Annu. Rev. Biochem. 1980;49:253–279. - PubMed
-
- Bjornberg O., Rowland P., Larsen S., Jensen K.F. Active site of dihydroorotate dehydrogenase A from Lactococcus lactis investigated by chemical modification and mutagenesis. Biochemistry. 1997;36:16197–16205. - PubMed
-
- Rowland P., Nielsen F.S., Jensen K.F., Larse S. The crystal structure of the flavin containing enzyme dihydroorotate dehydrogenase A from Lactococcus lactis. Structure. 1997;5:239–252. - PubMed
-
- Dimitrijevic M., Bartlett R.R. Leflunomide, a novel immunomodulating drug, inhibits homotypic adhesion of peripheral blood and synovial fluid mononuclear cells in rheumatoid arthritis. Inflamm. Res. 1996;45:550–556. - PubMed
-
- Smolen J.S., Kalden J.R., Scott D.L., Rozman B., Kvien T.K., Larsen A., Loew-Friedrich I., Oed C., Rosenburg R. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. Lancet. 1999;353:259–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

