Membrane-induced folding and structure of membrane-bound annexin A1 N-terminal peptides: implications for annexin-induced membrane aggregation
- PMID: 17993484
- PMCID: PMC2242736
- DOI: 10.1529/biophysj.107.119685
Membrane-induced folding and structure of membrane-bound annexin A1 N-terminal peptides: implications for annexin-induced membrane aggregation
Abstract
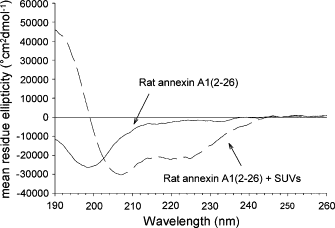
Annexins constitute a family of calcium-dependent membrane-binding proteins and can be classified into two groups, depending on the length of the N-terminal domain unique for each individual annexin. The N-terminal domain of annexin A1 can adopt an alpha-helical conformation and has been implicated in mediating the membrane aggregation behavior of this protein. Although the calcium-independent interaction of the annexin A1 N-terminal domain has been known for some time, there was no structural information about the membrane interaction of this secondary membrane-binding site of annexin A1. This study used circular dichroism spectroscopy to show that a rat annexin A1 N-terminal peptide possesses random coil structure in aqueous buffer but an alpha-helical structure in the presence of small unilamellar vesicles. The binding of peptides to membranes was confirmed by surface pressure (Langmuir film balance) measurements using phosphatidylcholine/phosphatidylserine monolayers, which show a significant increase after injection of rat annexin A1 N-terminal peptides. Lamellar neutron diffraction with human and rat annexin A1 N-terminal peptides reveals an intercalation of the helical peptides with the phospholipid bilayer, with the helix axis lying parallel to the surface of membrane. Our findings confirm that phospholipid membranes assist the folding of the N-terminal peptides into alpha-helical structures and that this conformation enables favorable direct interactions with the membrane. The results are consistent with the hypothesis that the N-terminal domain of annexin A1 can serve as a secondary membrane binding site in the process of membrane aggregation by providing a peripheral membrane anchor.
Figures




References
-
- Gerke V., Creutz C.E., Moss S.E. Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6:449–461. - PubMed
-
- Gerke V., Moss S.E. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. - PubMed
-
- Klee C.B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988;27:6645–6653. - PubMed
-
- Burgoyne R.D., Geisow M.J. The annexin family of calcium-binding proteins. Cell Calcium. 1989;10:1–10. - PubMed
-
- Swairjo M.A., Seaton B.A. Annexin structure and membrane interactions: a molecular perspective. Annu. Rev. Biophys. Bioeng. 1994;23:193–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

