Raft composition at physiological temperature and pH in the absence of detergents
- PMID: 17993486
- PMCID: PMC2267110
- DOI: 10.1529/biophysj.107.118596
Raft composition at physiological temperature and pH in the absence of detergents
Abstract
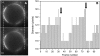
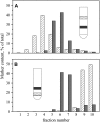
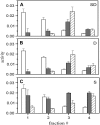
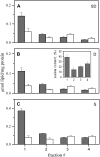
Biological rafts were identified and isolated at 37 degrees C and neutral pH. The strategy for isolating rafts utilized membrane tension to generate large domains. For lipid compositions that led only to microscropically unresolvable rafts in lipid bilayers, membrane tension led to the appearance of large, observable rafts. The large rafts converted back to small ones when tension was relieved. Thus, tension reversibly controls raft enlargement. For cells, application of membrane tension resulted in several types of large domains; one class of the domains was identified as rafts. Tension was generated in several ways, and all yielded raft fractions that had essentially the same composition, validating the principle of tension as a means to merge small rafts into large rafts. It was demonstrated that sphingomyelin-rich vesicles do not rise during centrifugation in sucrose gradients because they resist lysis, necessitating that, contrary to current experimental practice, membrane material be placed toward the top of a gradient for raft fractionation. Isolated raft fractions were enriched in a GPI-linked protein, alkaline phosphatase, and were poor in Na(+)-K(+) ATPase. Sphingomyelin and gangliosides were concentrated in rafts, the expected lipid raft composition. Cholesterol, however, was distributed equally between raft and nonraft fractions, contrary to the conventional view.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

