Theoretical models of eumelanin protomolecules and their optical properties
- PMID: 17993493
- PMCID: PMC2257886
- DOI: 10.1529/biophysj.107.121087
Theoretical models of eumelanin protomolecules and their optical properties
Abstract
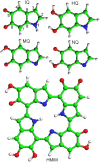
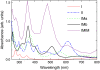
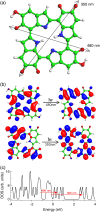
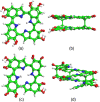
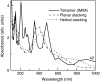
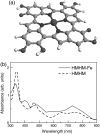
The molecular structure of melanin, one of the most ubiquitous natural pigments in living organisms, is not known and its multifaceted biological role is still debated. We examine structural models for eumelanin protomolecules, based on tetramers consisting of four monomer units (hydroquinone, indolequinone, and its two tautomers), in arrangements that contain an interior porphyrin ring. These models reproduce convincingly many aspects of eumelanin's experimentally observed behavior. In particular, we present a plausible synthetic pathway of the tetramers and their further complexation through interlayer stacking, or through formation of helical superstructures, into eumelanin macromolecules. The unsaturated nature of C-C bonds in indolequinone units and the finite size of protomolecules introduce covalent bond formation between stacked layers. We employ time-dependent density functional theory to calculate the optical absorption spectrum of each molecule along the eumelanin synthesis pathway, which gradually develops into the characteristic broad-band adsorption of melanin pigment due to electron delocalization. These optical spectra may serve as signatures for identifying intermediate structures.
Figures


Similar articles
-
Structural model of eumelanin.Phys Rev Lett. 2006 Nov 24;97(21):218102. doi: 10.1103/PhysRevLett.97.218102. Epub 2006 Nov 21. Phys Rev Lett. 2006. PMID: 17155775
-
Clue to Understanding the Janus Behavior of Eumelanin: Investigating the Relationship between Hierarchical Assembly Structure of Eumelanin and Its Photophysical Properties.Biomacromolecules. 2016 Sep 12;17(9):2860-72. doi: 10.1021/acs.biomac.6b00686. Epub 2016 Aug 9. Biomacromolecules. 2016. PMID: 27459629
-
Evidence of Porphyrin-Like Structures in Natural Melanin Pigments Using Electrochemical Fingerprinting.Adv Mater. 2016 Apr;28(16):3173-80. doi: 10.1002/adma.201504650. Epub 2016 Feb 29. Adv Mater. 2016. PMID: 26924536
-
Elucidating the Structure of Melanin and Its Structure-Property Correlation.Acc Chem Res. 2025 May 6;58(9):1509-1518. doi: 10.1021/acs.accounts.5c00120. Epub 2025 Apr 15. Acc Chem Res. 2025. PMID: 40232311 Review.
-
Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution.Int J Mol Sci. 2016 Apr 8;17(4):520. doi: 10.3390/ijms17040520. Int J Mol Sci. 2016. PMID: 27070583 Free PMC article. Review.
Cited by
-
Fast and Reliable Synthesis of Melanin Nanoparticles with Fine-Tuned Metal Adsorption Capacities for Studying Heavy Metal Ions Uptake.Nanotechnol Sci Appl. 2021 May 24;14:101-111. doi: 10.2147/NSA.S296722. eCollection 2021. Nanotechnol Sci Appl. 2021. PMID: 34079238 Free PMC article.
-
Optimization of Bacillus licheniformis MAL tyrosinase: in vitro anticancer activity for brown and black eumelanin.Heliyon. 2019 May 8;5(5):e01657. doi: 10.1016/j.heliyon.2019.e01657. eCollection 2019 May. Heliyon. 2019. PMID: 31111112 Free PMC article.
-
The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities.Int J Mol Sci. 2018 Jun 13;19(6):1753. doi: 10.3390/ijms19061753. Int J Mol Sci. 2018. PMID: 29899264 Free PMC article. Review.
-
Solid-state NMR Reveals the Carbon-based Molecular Architecture of Cryptococcus neoformans Fungal Eumelanins in the Cell Wall.J Biol Chem. 2015 May 29;290(22):13779-90. doi: 10.1074/jbc.M114.618389. Epub 2015 Mar 30. J Biol Chem. 2015. PMID: 25825492 Free PMC article.
-
Mechanisms for ultrafast nonradiative relaxation in electronically excited eumelanin constituents.Biophys J. 2008 Nov 1;95(9):4396-402. doi: 10.1529/biophysj.108.135756. Epub 2008 Aug 1. Biophys J. 2008. PMID: 18676639 Free PMC article.
References
-
- Ito, S. 2003. A chemist's view of melanogenesis. Pigment Cell Res. 16:230–236. - PubMed
-
- Morison, W. L. 1985. What is the function of melanin? Arch. Dermatol. 121:1160–1163. - PubMed
-
- Hill, H. Z. 1992. The function of melanin. Bioessays. 14:49–56. - PubMed
-
- Kollias, N., R. M. Sayre, L. Zeise, and M. R. Chedekel. 1991. Photoprotection by melanin. J. Photochem. Photobiol. B. 9:135–160. - PubMed
-
- Ortonne, J.-P. 2002. Photoprotective properties of skin melanin. Br. J. Dermatol. 146:7–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

