Molecular origin of the self-assembly of lanreotide into nanotubes: a mutational approach
- PMID: 17993497
- PMCID: PMC2242760
- DOI: 10.1529/biophysj.107.108175
Molecular origin of the self-assembly of lanreotide into nanotubes: a mutational approach
Abstract
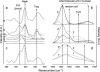
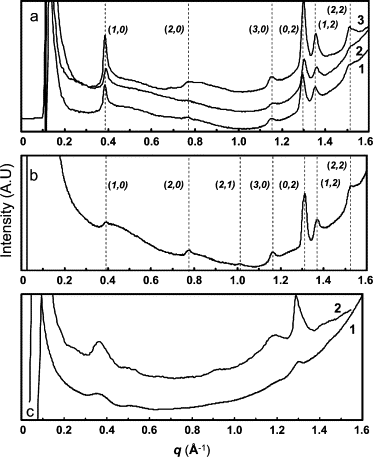
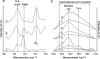
Lanreotide, a synthetic, therapeutic octapeptide analog of somatostatin, self-assembles in water into perfectly hollow and monodisperse (24-nm wide) nanotubes. Lanreotide is a cyclic octapeptide that contains three aromatic residues. The molecular packing of the peptide in the walls of a nanotube has recently been characterized, indicating four hierarchical levels of organization. This is a fascinating example of spontaneous self-organization, very similar to the formation of the gas vesicle walls of Halobacterium halobium. However, this unique peptide self-assembly raises important questions about its molecular origin. We adopted a directed mutation approach to determine the molecular parameters driving the formation of such a remarkable peptide architecture. We have modified the conformation by opening the cycle and by changing the conformation of a Lys residue, and we have also mutated the aromatic side chains of the peptide. We show that three parameters are essential for the formation of lanreotide nanotubes: i), the specificity of two of the three aromatic side chains, ii), the spatial arrangement of the hydrophilic and hydrophobic residues, and iii), the aromatic side chain in the beta-turn of the molecule. When these molecular characteristics are modified, either the peptides lose their self-assembling capability or they form less-ordered architectures, such as amyloid fibers and curved lamellae. Thus we have determined key elements of the molecular origins of lanreotide nanotube formation.
Figures






References
-
- Makin O.S., Serpell L.C. Structures for amyloid fibrils. FEBS J. 2005;272:5950–5961. - PubMed
-
- Papanikolopoulou K., Schoehn G., Forge V., Forsyth V.T., Riekel C., Hernandez J.F., Ruigrok R.W., Mitraki A. Amyloid fibril formation from sequences of a natural β-structured fibrous protein, the adenovirus fiber. J. Biol. Chem. 2005;280:2481–2490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

