Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro
- PMID: 17993501
- PMCID: PMC2212694
- DOI: 10.1529/biophysj.106.098574
Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro
Abstract
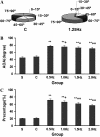
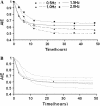
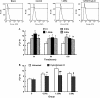
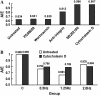
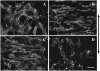
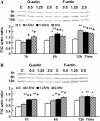
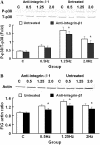
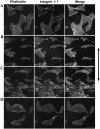
The arterial system is subjected to cyclic strain because of periodic alterations in blood pressure, but the effects of frequency of cyclic strain on arterial smooth muscle cells (SMCs) remain unclear. Here, we investigated the potential role of the cyclic strain frequency in regulating SMC alignment using an in vitro model. Aortic SMCs were subject to cyclic strain at one elongation but at various frequencies using a Flexercell Tension Plus system. It was found that the angle information entropy, the activation of integrin-beta1, p38 MAPK, and F/G actin ratio of filaments were all changed in a frequency-dependent manner, which was consistent with SMC alignment under cyclic strain with various frequencies. A treatment with anti-integrin-beta1 antibody, SB202190, or cytochalasin D inhibited the cyclic strain frequency-dependent SMC alignment. These observations suggested that the frequency of cyclic strain plays a role in regulating the alignment of vascular SMCs in an intact actin filament-dependent manner, and cyclic strain at 1.25 Hz was the most effective frequency influencing SMC alignment. Furthermore, integrin-beta1 and p38 MAPK possibly mediated cyclic strain frequency-dependent SMC alignment.
Figures

References
-
- Tranquillo, R. T., T. S. Girton, B. A. Bromberek, T. G. Triebes, and D. L. Mooradian. 1996. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials. 17:349–357. - PubMed
-
- Finlay, H. M., P. Whittaker, and P. B. Canham. 1998. Collagen organization in the branching region of human brain arteries. Stroke. 29:1595–1601. - PubMed
-
- L'Heureux, N., L. Germain, R. Labbe, and F. A. Auger. 1993. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J. Vasc. Surg. 17:499–509. - PubMed
-
- Standley, P. R., A. Cammarata, B. P. Nolan, C. T. Purgason, and M. A. Stanley. 2002. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am. J. Physiol. Heart Circ. Physiol. 283:H1907–H1914. - PubMed
-
- Kanda, K., and T. Matsuda. 1993. Behavior of arterial wall cells cultured on periodically stretched substrates. Cell Transplant. 2:475–484. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

