Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria: cell plan, glycogen storage, and localization of cytochrome C proteins
- PMID: 17993524
- PMCID: PMC2223682
- DOI: 10.1128/JB.01449-07
Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria: cell plan, glycogen storage, and localization of cytochrome C proteins
Abstract
Anaerobic ammonium oxidation (anammox) is an ecologically and industrially important process and is performed by a clade of deeply branching Planctomycetes. Anammox bacteria possess an intracytoplasmic membrane-bounded organelle, the anammoxosome. In the present study, the ultrastructures of four different genera of anammox bacteria were compared with transmission electron microscopy and electron tomography. The four anammox genera shared a common cell plan and contained glycogen granules. Differences between the four genera included cell size (from 800 to 1,100 nm in diameter), presence or absence of cytoplasmic particles, and presence or absence of pilus-like appendages. Furthermore, cytochrome c proteins were detected exclusively inside the anammoxosome. This detection provides further support for the hypothesis that this organelle is the locus of anammox catabolism.
Figures
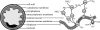
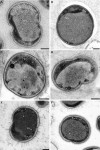

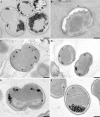
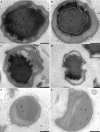
References
-
- Arp, D. J., and L. Y. Stein. 2003. Metabolism of inorganic N compounds by ammonium-oxidizing bacteria. Crit. Rev. Biochem. Mol. Biol. 38471-495. - PubMed
-
- Averill, B. A. 1996. Dissimilatory nitrite and nitric oxide reductases. Chem. Rev. 962951-2964. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1462. - PubMed
-
- Bonafonte, M. A., C. Solano, B. Sesma, M. Alvarez, L. Montuenga, D. García-Ros, and C. Gamazo. 2000. The relationship between glycogen synthesis, biofilm formation and virulence in Salmonella enteritidis. FEMS Microbiol. Lett. 19131-36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

