Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri
- PMID: 17993531
- PMCID: PMC2223550
- DOI: 10.1128/JB.01417-07
Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri
Abstract
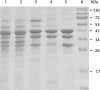
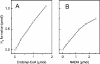
Cell extracts of butyrate-forming clostridia have been shown to catalyze acetyl-coenzyme A (acetyl-CoA)- and ferredoxin-dependent formation of H2 from NADH. It has been proposed that these bacteria contain an NADH:ferredoxin oxidoreductase which is allosterically regulated by acetyl-CoA. We report here that ferredoxin reduction with NADH in cell extracts from Clostridium kluyveri is catalyzed by the butyryl-CoA dehydrogenase/Etf complex and that the acetyl-CoA dependence previously observed is due to the fact that the cell extracts catalyze the reduction of acetyl-CoA with NADH via crotonyl-CoA to butyryl-CoA. The cytoplasmic butyryl-CoA dehydrogenase complex was purified and is shown to couple the endergonic reduction of ferredoxin (E0' = -410 mV) with NADH (E0' = -320 mV) to the exergonic reduction of crotonyl-CoA to butyryl-CoA (E0' = -10 mV) with NADH. The stoichiometry of the fully coupled reaction is extrapolated to be as follows: 2 NADH + 1 oxidized ferredoxin + 1 crotonyl-CoA = 2 NAD+ + 1 ferredoxin reduced by two electrons + 1 butyryl-CoA. The implications of this finding for the energy metabolism of butyrate-forming anaerobes are discussed in the accompanying paper.
Figures

Comment in
-
Classic Spotlight: Electron Bifurcation, a Unifying Concept for Energy Conservation in Anaerobes.J Bacteriol. 2016 Apr 14;198(9):1358. doi: 10.1128/JB.00185-16. Print 2016 May. J Bacteriol. 2016. PMID: 27080054 Free PMC article. No abstract available.
Similar articles
-
Reduction of ferredoxin or oxygen by flavin-based electron bifurcation in Megasphaera elsdenii.FEBS J. 2015 Aug;282(16):3149-60. doi: 10.1111/febs.13308. Epub 2015 May 18. FEBS J. 2015. PMID: 25903584
-
NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri.J Bacteriol. 2010 Oct;192(19):5115-23. doi: 10.1128/JB.00612-10. Epub 2010 Jul 30. J Bacteriol. 2010. PMID: 20675474 Free PMC article.
-
Reduction of Flavodoxin by Electron Bifurcation and Sodium Ion-dependent Reoxidation by NAD+ Catalyzed by Ferredoxin-NAD+ Reductase (Rnf).J Biol Chem. 2016 Jun 3;291(23):11993-2002. doi: 10.1074/jbc.M116.726299. Epub 2016 Apr 5. J Biol Chem. 2016. PMID: 27048649 Free PMC article.
-
Flavin-Based Electron Bifurcation, Ferredoxin, Flavodoxin, and Anaerobic Respiration With Protons (Ech) or NAD+ (Rnf) as Electron Acceptors: A Historical Review.Front Microbiol. 2018 Mar 14;9:401. doi: 10.3389/fmicb.2018.00401. eCollection 2018. Front Microbiol. 2018. PMID: 29593673 Free PMC article. Review.
-
Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation.Biochim Biophys Acta. 2013 Feb;1827(2):94-113. doi: 10.1016/j.bbabio.2012.07.002. Epub 2012 Jul 16. Biochim Biophys Acta. 2013. PMID: 22800682 Review.
Cited by
-
In vitro prototyping and rapid optimization of biosynthetic enzymes for cell design.Nat Chem Biol. 2020 Aug;16(8):912-919. doi: 10.1038/s41589-020-0559-0. Epub 2020 Jun 15. Nat Chem Biol. 2020. PMID: 32541965
-
An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis.PLoS One. 2012;7(3):e33439. doi: 10.1371/journal.pone.0033439. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479398 Free PMC article.
-
Microbiome-informed study of the mechanistic basis of methane inhibition by Asparagopsis taxiformis in dairy cattle.mBio. 2024 Aug 14;15(8):e0078224. doi: 10.1128/mbio.00782-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953639 Free PMC article.
-
Clostridium sticklandii, a specialist in amino acid degradation:revisiting its metabolism through its genome sequence.BMC Genomics. 2010 Oct 11;11:555. doi: 10.1186/1471-2164-11-555. BMC Genomics. 2010. PMID: 20937090 Free PMC article.
-
In vitro kinetics of prebiotic inulin-type fructan fermentation by butyrate-producing colon bacteria: implementation of online gas chromatography for quantitative analysis of carbon dioxide and hydrogen gas production.Appl Environ Microbiol. 2009 Sep;75(18):5884-92. doi: 10.1128/AEM.00876-09. Epub 2009 Jul 24. Appl Environ Microbiol. 2009. PMID: 19633122 Free PMC article.
References
-
- Alber, B. E., R. Spanheimer, C. Ebenau-Jehle, and G. Fuchs. 2006. Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol. Microbiol. 61297-309. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. - PubMed
-
- Chen, J. S., and L. E. Mortenson. 1974. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim. Biophys. Acta 371283-298. - PubMed
-
- Darrouzet, E., J. W. Cooley, and F. Daldal. 2004. The cytochrome bc1 complex and its homologue the b6 f complex: similarities and differences. Photosynth. Res. 7925-44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

