Campylobacter jejuni biofilms up-regulated in the absence of the stringent response utilize a calcofluor white-reactive polysaccharide
- PMID: 17993532
- PMCID: PMC2223549
- DOI: 10.1128/JB.00516-07
Campylobacter jejuni biofilms up-regulated in the absence of the stringent response utilize a calcofluor white-reactive polysaccharide
Abstract
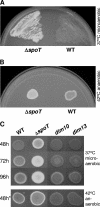
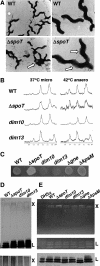
The enteric pathogen Campylobacter jejuni is a highly prevalent yet fastidious bacterium. Biofilms and surface polysaccharides participate in stress survival, transmission, and virulence in C. jejuni; thus, the identification and characterization of novel genes involved in each process have important implications for pathogenesis. We found that C. jejuni reacts with calcofluor white (CFW), indicating the presence of surface polysaccharides harboring beta1-3 and/or beta1-4 linkages. CFW reactivity increased with extended growth, under 42 degrees C anaerobic conditions, and in a DeltaspoT mutant defective for the stringent response (SR). Conversely, two newly isolated dim mutants exhibited diminished CFW reactivity as well as growth and serum sensitivity differences from the wild type. Genetic, biochemical, and nuclear magnetic resonance analyses suggested that differences in CFW reactivity between wild-type and DeltaspoT and dim mutant strains were independent of well-characterized lipooligosaccharides, capsular polysaccharides, and N-linked polysaccharides. Targeted deletion of carB downstream of the dim13 mutation also resulted in CFW hyporeactivity, implicating a possible role for carbamoylphosphate synthase in the biosynthesis of this polysaccharide. Correlations between biofilm formation and production of the CFW-reactive polymer were demonstrated by crystal violet staining, scanning electron microscopy, and confocal microscopy, with the C. jejuni DeltaspoT mutant being the first SR mutant in any bacterial species identified as up-regulating biofilms. Together, these results provide new insight into genes and processes important for biofilm formation and polysaccharide production in C. jejuni.
Figures

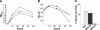
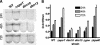

Similar articles
-
Effects of sequential Campylobacter jejuni 81-176 lipooligosaccharide core truncations on biofilm formation, stress survival, and pathogenesis.J Bacteriol. 2010 Apr;192(8):2182-92. doi: 10.1128/JB.01222-09. Epub 2010 Feb 5. J Bacteriol. 2010. PMID: 20139192 Free PMC article.
-
Temporal dynamics of gene expression during the development of Campylobacter jejuni biofilms.Microb Genom. 2025 May;11(5):001387. doi: 10.1099/mgen.0.001387. Microb Genom. 2025. PMID: 40327030 Free PMC article.
-
The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes.Mol Microbiol. 2005 Apr;56(1):8-27. doi: 10.1111/j.1365-2958.2005.04525.x. Mol Microbiol. 2005. PMID: 15773975
-
Molecules Autoinducer 2 and cjA and Their Impact on Gene Expression in Campylobacter jejuni.J Mol Microbiol Biotechnol. 2018;28(5):207-215. doi: 10.1159/000495411. Epub 2019 Jan 29. J Mol Microbiol Biotechnol. 2018. PMID: 30695777 Review.
-
Does Campylobacter jejuni form biofilms in food-related environments?Appl Environ Microbiol. 2014 Sep;80(17):5154-60. doi: 10.1128/AEM.01493-14. Epub 2014 Jun 13. Appl Environ Microbiol. 2014. PMID: 24928882 Free PMC article. Review.
Cited by
-
Virulence and Genomic Feature of Multidrug Resistant Campylobacter jejuni Isolated from Broiler Chicken.Front Microbiol. 2016 Oct 14;7:1605. doi: 10.3389/fmicb.2016.01605. eCollection 2016. Front Microbiol. 2016. PMID: 27790202 Free PMC article.
-
Biofilm formation by Campylobacter jejuni is increased under aerobic conditions.Appl Environ Microbiol. 2010 Apr;76(7):2122-8. doi: 10.1128/AEM.01878-09. Epub 2010 Feb 5. Appl Environ Microbiol. 2010. PMID: 20139307 Free PMC article.
-
Polyphosphate kinase 2: a novel determinant of stress responses and pathogenesis in Campylobacter jejuni.PLoS One. 2010 Aug 17;5(8):e12142. doi: 10.1371/journal.pone.0012142. PLoS One. 2010. PMID: 20808906 Free PMC article.
-
Enhanced Biofilm Formation by Ferrous and Ferric Iron Through Oxidative Stress in Campylobacter jejuni.Front Microbiol. 2018 Jun 6;9:1204. doi: 10.3389/fmicb.2018.01204. eCollection 2018. Front Microbiol. 2018. PMID: 29928267 Free PMC article.
-
Morphology heterogeneity within a Campylobacter jejuni helical population: the use of calcofluor white to generate rod-shaped C. jejuni 81-176 clones and the genetic determinants responsible for differences in morphology within 11168 strains.Mol Microbiol. 2017 Jun;104(6):948-971. doi: 10.1111/mmi.13672. Epub 2017 Apr 24. Mol Microbiol. 2017. PMID: 28316093 Free PMC article.
References
-
- Aspinall, G. O., A. G. McDonald, and H. Pang. 1992. Structures of the O chains from lipopolysaccharides of Campylobacter jejuni serotypes O:23 and O:36. Carbohydr. Res. 23113-30. - PubMed
-
- Aspinall, G. O., A. G. McDonald, T. S. Raju, H. Pang, A. P. Moran, and J. L. Penner. 1993. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur. J. Biochem. 2131017-1027. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1995. Current protocols in molecular biology, Wiley Interscience, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

