The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth
- PMID: 17993544
- PMCID: PMC2230571
- DOI: 10.1104/pp.107.109058
The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth
Abstract
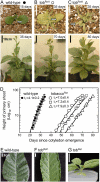
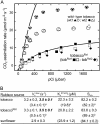
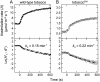
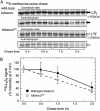
Plastomic replacement of the tobacco (Nicotiana tabacum) Rubisco large subunit gene (rbcL) with that from sunflower (Helianthus annuus; rbcL(S)) produced tobacco(Rst) transformants that produced a hybrid Rubisco consisting of sunflower large and tobacco small subunits (L(s)S(t)). The tobacco(Rst) plants required CO(2) (0.5% v/v) supplementation to grow autotrophically from seed despite the substrate saturated carboxylation rate, K(m), for CO(2) and CO(2)/O(2) selectivity of the L(s)S(t) enzyme mirroring the kinetically equivalent tobacco and sunflower Rubiscos. Consequently, at the onset of exponential growth when the source strength and leaf L(s)S(t) content were sufficient, tobacco(Rst) plants grew to maturity without CO(2) supplementation. When grown under a high pCO(2), the tobacco(Rst) seedlings grew slower than tobacco and exhibited unique growth phenotypes: Juvenile plants formed clusters of 10 to 20 structurally simple oblanceolate leaves, developed multiple apical meristems, and the mature leaves displayed marginal curling and dimpling. Depending on developmental stage, the L(s)S(t) content in tobacco(Rst) leaves was 4- to 7-fold less than tobacco, and gas exchange coupled with chlorophyll fluorescence showed that at 2 mbar pCO(2) and growth illumination CO(2) assimilation in mature tobacco(Rst) leaves remained limited by Rubisco activity and its rate (approximately 11 micromol m(-2) s(-1)) was half that of tobacco controls. (35)S-methionine labeling showed the stability of assembled L(s)S(t) was similar to tobacco Rubisco and measurements of light transient CO(2) assimilation rates showed L(s)S(t) was adequately regulated by tobacco Rubisco activase. We conclude limitations to tobacco(Rst) growth primarily stem from reduced rbcL(S) mRNA levels and the translation and/or assembly of sunflower large with the tobacco small subunits that restricted L(s)S(t) synthesis.
Figures



References
-
- Andrews TJ, Whitney SM (2003) Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of higher plants. Arch Biochem Biophys 414 159–169 - PubMed
-
- Bollenbach TJ, Schuster G, Stern DB (2004) Cooperation of endo- and exoribonucleases in chloroplast mRNA turnover. In K Moldave, ed, Progress in Nucleic Acid Research and Molecular Biology, Vol 78. Elsevier Academic Press, Amsterdam, pp 305–337 - PubMed
-
- Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase oxygenase and the rate of respiration in the light—estimates from gas-exchange measurements on spinach. Planta 165 397–406 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

