Involvement of polyamine oxidase in wound healing
- PMID: 17993545
- PMCID: PMC2230557
- DOI: 10.1104/pp.107.108902
Involvement of polyamine oxidase in wound healing
Abstract
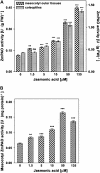
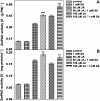
Hydrogen peroxide (H(2)O(2)) is involved in plant defense responses that follow mechanical damage, such as those that occur during herbivore or insect attacks, as well as pathogen attack. H(2)O(2) accumulation is induced during wound healing processes as well as by treatment with the wound signal jasmonic acid. Plant polyamine oxidases (PAOs) are H(2)O(2) producing enzymes supposedly involved in cell wall differentiation processes and defense responses. Maize (Zea mays) PAO (ZmPAO) is a developmentally regulated flavoprotein abundant in primary and secondary cell walls of several tissues. In this study, we investigated the effect of wounding on ZmPAO gene expression in the outer tissues of the maize mesocotyl and provide evidence that ZmPAO enzyme activity, protein, and mRNA levels increased in response to wounding as well as jasmonic acid treatment. Histochemically detected ZmPAO activity especially intensified in the epidermis and in the wound periderm, suggesting a tissue-specific involvement of ZmPAO in wound healing. The role played by ZmPAO-derived H(2)O(2) production in peroxidase-mediated wall stiffening events was further investigated by exploiting the in vivo use of N-prenylagmatine (G3), a selective and powerful ZmPAO inhibitor, representing a reliable diagnostic tool in discriminating ZmPAO-mediated H(2)O(2) production from that generated by peroxidase, oxalate oxidase, or by NADPH oxidase activity. Here, we demonstrate that G3 inhibits wound-induced H(2)O(2) production and strongly reduces lignin and suberin polyphenolic domain deposition along the wound, while it is ineffective in inhibiting the deposition of suberin aliphatic domain. Moreover, ZmPAO ectopic expression in the cell wall of transgenic tobacco (Nicotiana tabacum) plants strongly enhanced lignosuberization along the wound periderm, providing evidence for a causal relationship between PAO and peroxidase-mediated events during wound healing.
Figures










References
-
- Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28 1867–1876 - PubMed
-
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55 373–399 - PubMed
-
- Artiss JD, Entwistle WM (1981) The application of a sensitive uricase-peroxidase couple reaction to a centrifugal fast analyser for the determination of uric acid. Clin Chim Acta 116 301–309 - PubMed
-
- Augeri M, Angelini R, Federico R (1990) Sub-cellular localization and tissue distribution of polyamine oxidase in maize (Zea mays L.) seedlings. J Plant Physiol 136 690–695
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

