Cloning and characterization of two Lactobacillus casei genes encoding a cystathionine lyase
- PMID: 17993563
- PMCID: PMC2223195
- DOI: 10.1128/AEM.00745-07
Cloning and characterization of two Lactobacillus casei genes encoding a cystathionine lyase
Abstract
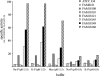
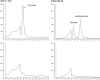
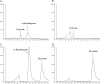
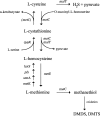
Volatile sulfur compounds are key flavor compounds in several cheese types. To better understand the metabolism of sulfur-containing amino acids, which certainly plays a key role in the release of volatile sulfur compounds, we searched the genome database of Lactobacillus casei ATCC 334 for genes encoding putative homologs of enzymes known to degrade cysteine, cystathionine, and methionine. The search revealed that L. casei possesses two genes that putatively encode a cystathionine beta-lyase (CBL; EC 4.4.1.8). The enzyme has been implicated in the degradation of not only cystathionine but also cysteine and methionine. Recombinant CBL proteins catalyzed the degradation of L-cystathionine, O-succinyl-L-homoserine, L-cysteine, L-serine, and L-methionine to form alpha-keto acid, hydrogen sulfide, or methanethiol. The two enzymes showed notable differences in substrate specificity and pH optimum.
Figures


References
-
- Ardö, Y. 2006. Flavour formation by amino acid catabolism. Biotechnol. Adv. 24:238-242. - PubMed
-
- Aubel, D., J. E. Germond, C. Gilbert, and D. Atlan. 2002. Isolation of the patC gene encoding the cystathionine β-lyase of Lactobacillus delbrueckii subsp. bulgaricus and molecular analysis of inter-strain variability in enzyme biosynthesis. Microbiology 148:2029-2036. - PubMed
-
- Auger, S., M. P. Gomez, A. Danchin, and I. Martin-Verstraete. 2005. The PatB protein of Bacillus subtilis is a C-S-lyase. Biochimie 87:231-238. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

