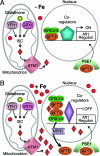
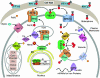
Response to iron deprivation in Saccharomyces cerevisiae
- PMID: 17993568
- PMCID: PMC2224162
- DOI: 10.1128/EC.00354-07
Response to iron deprivation in Saccharomyces cerevisiae
Figures
References
-
- Belli, G., M. M. Molina, J. Garcia-Martinez, J. E. Perez-Ortin, and E. Herrero. 2004. Saccharomyces cerevisiae glutaredoxin 5-deficient cells subjected to continuous oxidizing conditions are affected in the expression of specific sets of genes. J. Biol. Chem. 27912386-12395. - PubMed
-
- Blaiseau, P. L., E. Lesuisse, and J. M. Camadro. 2001. Aft2p, a novel iron-regulated transcription activator that modulates, with Aft1p, intracellular iron use and resistance to oxidative stress in yeast. J. Biol. Chem. 27634221-34226. - PubMed
-
- Burn, J., and P. F. Chinnery. 2006. Neuroferritinopathy. Semin. Pediatr. Neurol. 13176-181. - PubMed
-
- Chen, O. S., R. J. Crisp, M. Valachovic, M. Bard, D. R. Winge, and J. Kaplan. 2004. Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J. Biol. Chem. 27929513-29518. - PubMed
-
- Chen, O. S., and J. Kaplan. 2000. CCC1 suppresses mitochondrial damage in the yeast model of Friedreich's ataxia by limiting mitochondrial iron accumulation. J. Biol. Chem. 2757626-7632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

