Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility
- PMID: 17993573
- PMCID: PMC2224157
- DOI: 10.1128/EC.00301-07
Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility
Abstract
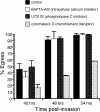
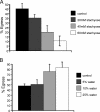
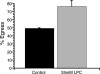
The process by which the intracellular parasite Toxoplasma gondii exits its host cell is central to its propagation and pathogenesis. Experimental induction of motility in intracellular parasites results in parasite egress, leading to the hypothesis that egress depends on the parasite's actin-dependent motility. Using a novel assay to monitor egress without experimental induction, we have established that inhibiting parasite motility does not block this process, although treatment with actin-disrupting drugs does delay egress. However, using an irreversible actin inhibitor, we show that this delay is due to the disruption of host cell actin alone, apparently resulting from the consequent loss of membrane tension. Accordingly, by manipulating osmotic pressure, we show that parasite egress is delayed by releasing membrane tension and promoted by increasing it. Therefore, without artificial induction, egress does not depend on parasite motility and can proceed by mechanical rupture of the host membrane.
Figures



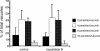


References
-
- Buitrago-Rey, R., J. Olarte, and J. E. Gomez-Marin. 2002. Evaluation of two inhibitors of invasion: LY311727 [3-(3-acetamide-1-benzyl-2-ethyl-indolyl-5-oxy)propane phosphonic acid] and AEBSF [4-(2-aminoethyl)-benzenesulphonyl fluoride] in acute murine toxoplasmosis. J. Antimicrob. Chemother. 49871-874. - PubMed
-
- Chernomordik, L. V., M. M. Kozlov, G. B. Melikyan, I. G. Abidor, V. S. Markin, and Y. A. Chizmadzhev. 1985. The shape of lipid molecules and monolayer membrane-fusion. Biochim. Biophys. Acta 812643-655.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

