Airway smooth muscle relaxation is impaired in mice lacking the p47phox subunit of NAD(P)H oxidase
- PMID: 17993584
- PMCID: PMC3391573
- DOI: 10.1152/ajplung.00384.2007
Airway smooth muscle relaxation is impaired in mice lacking the p47phox subunit of NAD(P)H oxidase
Retraction in
-
Retraction.Am J Physiol Lung Cell Mol Physiol. 2015 Apr 15;308(8):L854. doi: 10.1152/ajplung.zh5-6741-retr.2015. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25878295 Free PMC article. No abstract available.
Abstract
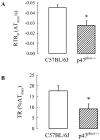
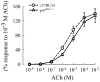
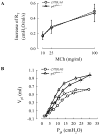
NAD(P)H oxidase is one of the critical enzymes mediating cellular production of reactive oxygen species and has a central role in airway smooth muscle (ASM) cell proliferation. Since reactive oxygen species also affect ASM contractile response, we hypothesized a regulatory role of NAD(P)H oxidase in ASM contractility. We therefore studied ASM function in wild-type mice (C57BL/6J) and mice deficient in a component (p47phox) of NAD(P)H oxidase. In histological sections of the trachea, we found that the area occupied by ASM was 17% more in p47(phox-/-) than in wild-type mice. After correcting for the difference in ASM content, we found that force generation did not vary between the two genotypes. Similarly, their ASM shortening velocity, maximal power, and sensitivity to acetylcholine, as well as airway responsiveness to methacholine in vivo, were not significantly different. The main finding of this study was a significantly reduced ASM relaxation in p47phox-/- compared with wild-type mice both during the stimulus and after the end of stimulation. The tension relaxation attained at the 20th second of electric field stimulation was, respectively, 17.6 +/- 2.4 and 9.2 +/- 2.3% in null and wild-type mice (P <0.01 by t-test). Similar significant differences were found in the rate of tension relaxation and the time required to reduce tension by one-half. Our data suggest that NAD(P)H oxidase may have a role in the structural arrangement and mechanical properties of the airway tissue. Most importantly, we report the first evidence that the p47phox subunit of NAD(P)H oxidase plays a role in ASM relaxation.
Figures






Comment in
-
Findings of Research Misconduct.Fed Regist. 2019 Nov 7;84(216):60097-60098. Fed Regist. 2019. PMID: 37547121 Free PMC article. No abstract available.
References
-
- Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274:33644–33653. - PubMed
-
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. - PMC - PubMed
-
- Antczak A, Nowak D, Shariati B, Krol M, Piasecka G, Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir J. 1997;10:1235–1241. - PubMed
-
- Bai TR. Abnormalities in airway smooth muscle in fatal asthma. A comparison between trachea and bronchus. Am Rev Respir Dis. 1991;143:441–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

