Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels
- PMID: 17993613
- PMCID: PMC2214740
- DOI: 10.1182/blood-2007-06-094318
Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels
Abstract
Tissue engineering requires formation of a de novo stable vascular network. Because of their ability to proliferate, differentiate into endothelial cells, and form new vessels, blood-derived endothelial progenitor cells (EPCs) are attractive source of cells for use in engineering blood vessels. However, the durability and function of EPC-derived vessels implanted in vivo are unclear. To this end, we directly compared formation and functions of tissue-engineered blood vessels generated by peripheral blood- and umbilical cord blood-derived EPCs in a model of in vivo vasculogenesis. We found that adult peripheral blood EPCs form blood vessels that are unstable and regress within 3 weeks. In contrast, umbilical cord blood EPCs form normal-functioning blood vessels that last for more than 4 months. These vessels exhibit normal blood flow, perm-selectivity to macromolecules, and induction of leukocyte-endothelial interactions in response to cytokine activation similar to normal vessels. Thus, umbilical cord blood EPCs hold great therapeutic potential, and their use should be pursued for vascular engineering.
Figures
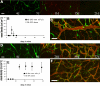
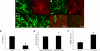
Similar articles
-
Capability of human umbilical cord blood progenitor-derived endothelial cells to form an efficient lining on a polyester vascular graft in vitro.Acta Biomater. 2009 May;5(4):1147-57. doi: 10.1016/j.actbio.2008.10.002. Epub 2008 Oct 17. Acta Biomater. 2009. PMID: 18996071
-
Human umbilical cord blood-derived CD34-positive endothelial progenitor cells stimulate osteoblastic differentiation of cultured human periosteal-derived osteoblasts.Tissue Eng Part A. 2014 Mar;20(5-6):940-53. doi: 10.1089/ten.TEA.2013.0329. Epub 2013 Dec 3. Tissue Eng Part A. 2014. PMID: 24168264
-
Immunological and ultrastructural characterization of endothelial cell cultures differentiated from human cord blood derived endothelial progenitor cells.Histochem Cell Biol. 2006 Dec;126(6):649-64. doi: 10.1007/s00418-006-0201-6. Epub 2006 Jun 10. Histochem Cell Biol. 2006. PMID: 16767408
-
Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors.Trends Cardiovasc Med. 2001 Nov;11(8):303-7. doi: 10.1016/s1050-1738(01)00128-1. Trends Cardiovasc Med. 2001. PMID: 11728877 Review.
-
Cord blood-derived early outgrowth endothelial progenitor cells.Microvasc Res. 2010 May;79(3):174-7. doi: 10.1016/j.mvr.2010.01.008. Epub 2010 Jan 18. Microvasc Res. 2010. PMID: 20085776 Review.
Cited by
-
Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood.J Vis Exp. 2012 Apr 13;(62):3872. doi: 10.3791/3872. J Vis Exp. 2012. PMID: 22526095 Free PMC article.
-
SDF-1α gene-activated collagen scaffold enhances provasculogenic response in a coculture of human endothelial cells with human adipose-derived stromal cells.J Mater Sci Mater Med. 2021 Mar 6;32(3):26. doi: 10.1007/s10856-021-06499-6. J Mater Sci Mater Med. 2021. PMID: 33677751 Free PMC article.
-
Cord blood-circulating endothelial progenitors for treatment of vascular diseases.Cell Prolif. 2011 Apr;44 Suppl 1(Suppl 1):44-7. doi: 10.1111/j.1365-2184.2010.00722.x. Cell Prolif. 2011. PMID: 21481043 Free PMC article.
-
Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion.Blood. 2011 Dec 15;118(25):6718-21. doi: 10.1182/blood-2011-08-375188. Epub 2011 Oct 28. Blood. 2011. PMID: 22039257 Free PMC article.
-
Non-hematopoietic stem cells in umbilical cord blood.Int J Stem Cells. 2009 May;2(2):83-9. doi: 10.15283/ijsc.2009.2.2.83. Int J Stem Cells. 2009. PMID: 24855525 Free PMC article.
References
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors [see comment]. Circulation. 2003;107:1164–1169. - PubMed
-
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. - PubMed
-
- Ott I, Keller U, Knoedler M, et al. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. Faseb J. 2005;19:992–994. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

